Understanding How T Cells Target Tuberculosis will Enhance Vaccines and Therapies

La Jolla Institute for Immunology (LJI) is working to guide the development of new tuberculosis vaccines and drug therapies. Now a team of LJI scientists has uncovered important clues to how human T cells combat Mycobacterium tuberculosis, the bacterium that causes TB. Their findings were published recently in Nature Communications.
“This research gives us a better understanding of T cell responses to different stages in tuberculosis infection and helps us figure out is there are additional diagnostic targets, vaccine targets, or drug candidates to help people with the disease,” says LJI Research Assistant Professor Cecilia Lindestam Arlehamn, PhD, who led the new research in collaboration with LJI Professors Bjoern Peters, PhD, and Alessandro Sette, Dr.Biol.Sci.
The urgent need for TB research
According to the World Health Organization, more than 1.3 million people died of TB in 2022, making it the second-leading infectious cause-of-death after COVID. “TB is a huge problem in many countries,” says Lindestam Arlehamn.
Currently, a vaccine called bacille Calmette-Guerin (BCG) protects against some severe cases of TB. Unfortunately, BCG doesn’t consistently prevent cases of pulmonary TB, which can also be deadly.
Although there are drug treatments for TB, more and more cases around the world have proven drug resistant.
To help stop TB, Lindestam Arlehamn and her colleagues are learning from T cells. Instead of targeting an entire pathogen, T cells look for specific markers, called peptides sequences, that belong to the pathogen.
When a T cell recognises a certain part of a pathogen’s peptide sequence, that area is termed an “epitope.”
Uncovering T cell epitopes gives scientists vital information on how vaccines and drug treatments might take aim at the same epitopes to stop a pathogen.
T cells take aim at a range of TB epitopes
For the new study, the researchers worked with samples from patients who were mid-treatment for active TB. These samples came from study participants in Peru, Sri Lanka, and Moldova.
By looking at T cells in patients from three different continents, the researchers hoped to capture a wide diversity of genetics and environmental factors that can affect immune system activity.
In their analysis, the LJI team uncovered 137 unique T cell epitopes. They found that 16% of these epitopes were targeted by T cells found in two or more patients. The immune system appeared to be working hard to zoom in on these epitopes.
Going forward, Lindestam Arlehamn’s laboratory will investigate which of these epitopes may be promising targets for future TB vaccines and drug therapies.
A step toward better diagnostics
The new study is also a step toward catching TB cases before they turn deadly.
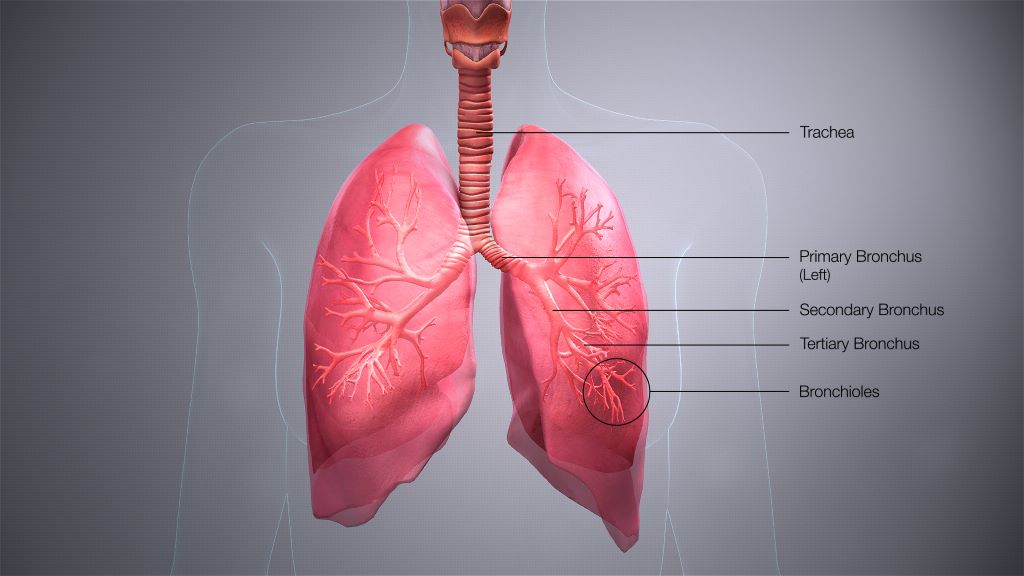
Because Mycobacterium tuberculosis is an airborne bacteria, a person can be exposed without ever realizing it. Once exposed, many people go months or years without any symptoms.
This inactive, or “latent,” TB can turn into active TB if a person’s immune system weakens, for example, during pregnancy or due to an infection such as HIV.
For the new study, the researchers also compared samples from active TB patients with samples from healthy individuals.
The scientists uncovered key differences in T cell reactivity between the two groups.
“For the first time, we could distinguish people with active TB versus those that have been exposed to TB – or unexposed individuals,” says Lindestam Arlehamn.
Lindestam Arlehamn says it may be possible to develop diagnostics that detect this tell-tale T cell reactivity that marks a person’s shift from latent to active TB. “Can we use this peptide pool to look for high-risk individuals and try and follow them over time?” she says.