Review Finds Little, if Any, Difference in Safety among COVID Vaccines
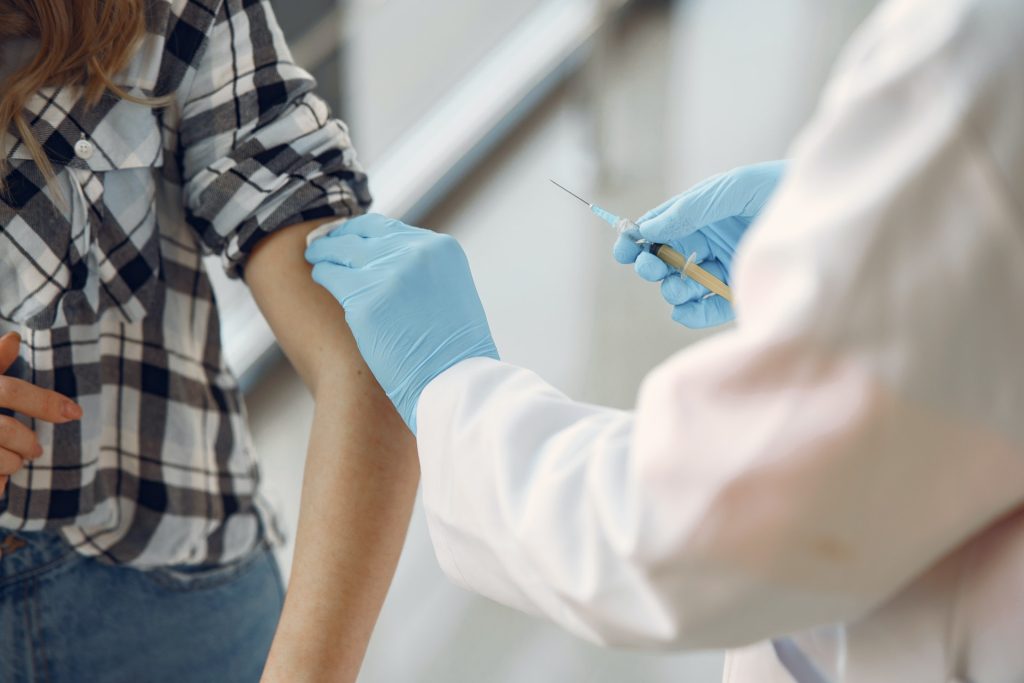
A Cochrane review of all the evidence available from randomised controlled trials of COVID vaccines up to November 2021 has concluded that most protect against infection and severe or critical illness caused by the virus. In addition, the Johnson and Johnson vaccine and the Cuban Soberana 2 vaccine “probably” reduced all-cause mortality.
The independent, international expert reviewers also found that there was little or no difference between the number of people experiencing serious side effects after vaccination compared to those who were unvaccinated.
The researchers, led by Isabelle Boutron, Professor of Epidemiology at Université Paris Cité and Director of Cochrane France, analysed published data from 41 randomised controlled trials of 12 different COVID vaccines, involving 433 838 people in various countries around the world. They assessed the certainty of the evidence and the risk of bias in the different studies.
The trials compared COVID vaccines with placebo, no vaccine, or each other, and were published before 5 November 2021. Most trials were no longer than two months in length.
The review found that the following vaccines reduced or probably reduced the risk of COVID infection compared to placebo: Pfizer/BioNTech, Moderna, CureVac COVID-19, Oxford-AstraZeneca, J&J, Sputnik V (Gam-COVID-Vac), Sinopharm (WIBP CorV and BBIBP-CorV), Bharat (Covaxin), Novavax and Soberana 2 (Finlay-FR-2). The following reduced or probably reduced the risk of severe or critical disease: Pfizer/BioNTech, Moderna, Janssen, Sputnik V, Bharat and Novavax. In addition, the J&J and Soberana 2 vaccines probably decreased the all-cause mortality risk. There were very few deaths recorded in all the trials and so evidence on mortality for the other vaccines is uncertain.
For most of the vaccines, vaccinated individuals reported more localised or temporary side effects compared no-treatment or placebo groups. These included tiredness, headache, muscle pains, chills, fever and nausea. With respect to the very rare side effects associated with some vaccines such as thrombosis, the team found that the reporting of these events was inconsistent, and the number of events reported in the trials was very low.
Given the evidence of efficacy of these vaccines, the researchers question whether further placebo-controlled trials are ethical. They suggest that further research compares new vaccines with those already in use.
Source: Wiley