Court Orders Gauteng Health Department to Treat Cancer Patients
Judge rules failure to deal with backlog of patients needing radiation treatment is unconstitutional
By Liezl Human

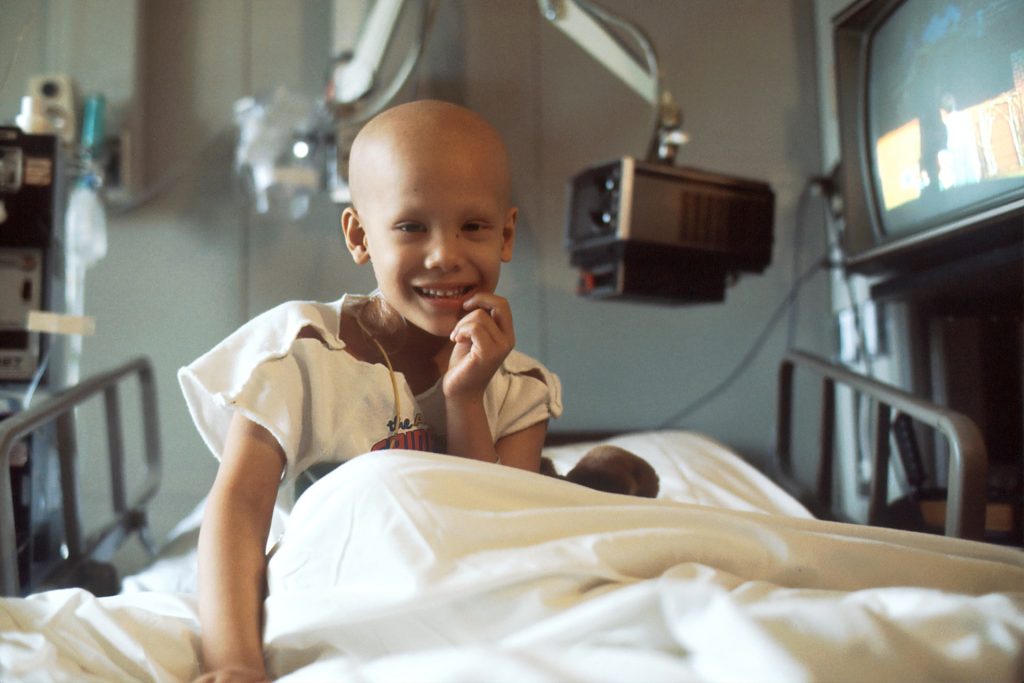
The Gauteng Department of Health is appealing against a judgment by the Johannesburg High Court ordering it to provide radiation oncology treatment to a backlog of nearly 3000 cancer patients at Charlotte Maxeke Hospital and Steve Biko Hospital.
In April last year, activists from SECTION27, Cancer Alliance and the Treatment Action Campaign (TAC) joined cancer patients to march to the department’s provincial office, demanding that millions of rands set aside for radiation treatment be used.
The matter was then taken to court by the Cancer Alliance, represented by SECTION27, after years of attempts to engage with the department about radiation services. They said in a statement they wanted the court to compel the department to provide treatment to the backlog of cancer patients still waiting.
Some patients have been on the list for nearly three years, while others have died while waiting, according to the judgment by Acting Judge Stephen van Nieuwenhuizen. He noted that “irreparable harm” has occurred and continues to occur in the absence of treatment.
He said the backlog, of mostly Charlotte Maxeke patients, had grown due to a lack of radiation equipment at the hospital and a shortage of staff. This was in spite of an allocation of R784-million over three years, specifically ring-fenced for radiology oncology services. The allocation was also meant to help clear the backlog of patients.
Delays in finalising a tender for these services meant that R250-million was returned to National Treasury at the end of the fiscal year, he said.
The judge found that the provincial department had infringed on the rights of these cancer patients in that a high standard of professional ethics had not been maintained. “Efficient and effective use of resources were not promoted. Services were not provided impartially, fairly, equitably and without bias.”
Judge van Nieuwenhuizen said the provincial health department had done “nothing meaningful” since the money was allocated in March 2023 to actually provide radiation oncology treatment to the cancer patients. “On the other hand, the health and general well-being of cancer patients has significantly deteriorated. There is a clear, imminent and ongoing irreparable harm that cancer patients who are on the backlog list are suffering.”
The judge ruled that the department’s failure to provide radiation services to cancer patients on the backlog list was unconstitutional and unlawful.
He added that the provincial health officials “have conducted themselves as a law unto themselves” and ordered that measures be put in place to ensure officials are “held to account for their constitutionally imposed obligation to provide healthcare services … to cancer patients who are on the backlog list”.
He also ordered that the list of cancer patients still awaiting radiation treatment must be updated within 45 days and that a progress report and long-term plan must be submitted to the court within three months.
Salomé Meyer, director of the Cancer Alliance, told GroundUp the ruling would allow the court to get accurate information on the circumstances of each patient still waiting for treatment.
She said the judgment “confirms that civil society has a role to play to hold the government responsible for what it is supposed to do”.
In a statement on 2 April, the department confirmed that it had filed an application for leave to appeal against the ruling. The department said “there are several substantive grounds of appeal, which if left unchallenged will be greatly prejudicial to the patients undergoing radiation oncology services at the hospitals” and might set an “undesirable” precedent.
Republished from Groundup under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Read the original article.