Leakiness of First Blood–brain Barrier Layer Results in Cognitive Deficits
A study published in Nature reveals how a key component of the blood–brain barrier (BBB), the endothelial glycocalyx layer, becomes dysregulated in ageing, causing the BBB to become compromised. The researchers also investigated the possibility of to restore this layer’s integrity, reducing neuroinflammation and restoring cognitive function.
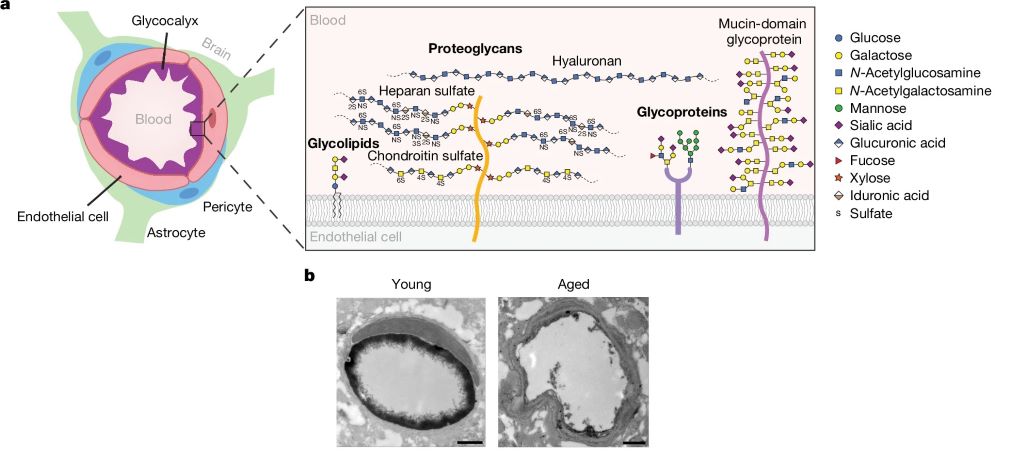
The BBB is a highly specialised safeguard keeping the brain separate from harmful factors, such as toxins and also albumin, IgG and fibrinogen (and, unfortunately, many medications which could otherwise treat brain disease). The leakage of such blood-derived molecules into the brain has been shown to trigger neuroinflammatory changes and create a neurotoxic brain environment. The part of the BBB directly in contact with the blood is the endothelial glycocalyx layer, a carbohydrate-rich meshwork mostly composed of proteoglycans, glycoproteins and glycolipids that coats the BBB lumen. Yet the endothelial glycocalyx’s composition and role is poorly understood despite it being the first layer of interface between the blood and brain.
The researchers found that the brain endothelial glycocalyx is highly dysregulated during ageing and neurodegenerative disease. Two mucin-type O-glycan biosynthetic enzymes, C1GALT1 and B3GNT3 were also found to be downregulated mouse models of ageing and in the brains of Alzheimer’s and Huntington’s disease patients. To test these, the researchers used adeno-associated viruses (AAV) in young mice to turn down the expression of C1GALT1 and B3GNT3. These mice showed signs of BBB leakage and in severe cases, brain haemorrhaging occurred in mice.
In samples from the brains of Alzheimer’s patients, the researchers also observed reduced C1GALT1 in microvessels.
To test if it was possible to restore the BBB’s ability to protect the brain against harmful blood-borne molecules, they administered AAVs in aged mice to restore levels of B3GNT3 and C1GALT1.
Assessing cognitive function, they found that aged mice treated with B3GNT3 via an AAV displayed improvements in spatial working memory in a maze test and hippocampal-dependent learning and memory in a fear conditioning test. Aged mice treated with C1GALT1 did not improve in the maze test, and no significant difference was observed in cued freezing in the fear conditioning among the three aged groups.
Although the study shows that increasing C1GALT1 and B3GNT3 reduces BBB permeability and improves brain health, the precise mechanisms that underlie these beneficial effects remain unclear. The researchers believe that by limiting the nonspecific uptake of blood-derived molecules, the brain can be protected. But C1GALT1 and B3GNT3 are also likely to influence a wide range of proteins and glycan structures and in order to further understand brain ageing and rejuvenation it is therefore crucial to understand the molecular pathways affected by them.
The authors concluded: “Cumulatively, our findings provide a detailed compositional and structural mapping of the ageing brain endothelial glycocalyx layer and reveal important consequences of ageing- and disease-associated glycocalyx dysregulation on BBB integrity and brain health.”