Chemists Discover Alarming Resistance of P. Aeruginosa to Common Cleaners

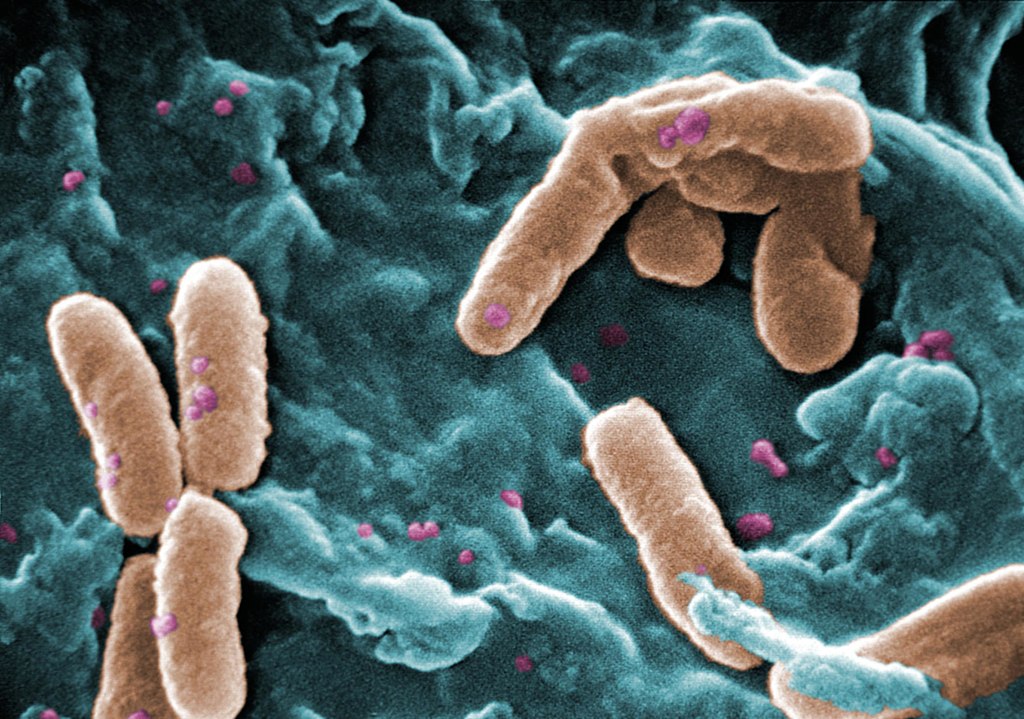
A new study reveals widespread resistance of a major bacterial pathogen to the active ingredients in cleaning agents commonly used in hospitals and homes. The American Chemical Society Infectious Diseases published the research led by chemists at Emory University. It demonstrates the surprising level of resistance to cleaning agents of multidrug-resistant Pseudomonas aeruginosa, a pathogen of particular concern in hospital settings.
The study also identifies biocides that are highly effective against P. aeruginosa, including a novel compound developed at Emory in collaboration with Villanova University. The researchers describe how these biocides work differently than most disinfectants currently in use.
“We hope our findings can help guide hospitals to reconsider protocols for the sanitation of patient rooms and other facilities,” says William Wuest, Emory professor of chemistry and a senior author of the study. “We also hope that our findings of a new mechanism of action against these bacterial strains may help in the design of future disinfectant products.”
First authors of the study are Christian Sanchez (who did the work as an Emory PhD student in chemistry and, following graduation, joined the faculty at Samford University) and German Vargas-Cuebas, an Emory PhD candidate in microbiology through Laney Graduate School.
“Resistance of pathogens to cleaning agents is an area that’s often overlooked,” Vargas-Cuebas says, “but it’s an important area of study, especially with the rise in antibiotic-resistant pathogens worldwide.”
Kevin Minbiole, professor of chemistry at Villanova, is co-senior author of the paper.
Workhorse disinfectants losing steam
Quaternary ammonium compounds, or QACs, are active ingredients commonly seen in household and hospital cleaners, including some disinfectant sprays and liquids, antibacterial sanitizing wipes and soaps.
“There are a handful of QACs that have been the workhorse disinfectants for around 100 years, on the frontline of most homes and hospitals,” Wuest says. “Very little has been done to modify their structures because they have long worked so well against many common bacteria, viruses, molds and fungi and they’re so simple and cheap to make.”
The Wuest lab is a leader in studies of QACs and other disinfecting agents. One issue Wuest and his colleagues have identified is that some bacterial strains are developing resistance to QACs. That trend could cause serious problems for sanitation in hospitals.
A pathogen of critical priority
More than 2.8 million antimicrobial-resistant infections occur in the United States each year, leading to more than 35,000 deaths, according to the Centers for Disease Control and Prevention (CDC).
The CDC names multidrug-resistant P. aeruginosa as one of seven pathogens causing infections that increased in the United States during the COVID-19 pandemic and remain above prepandemic levels.
Worldwide, P. aeruginosa causes more than 500,000 deaths annually and has been named a pathogen of critical priority by the World Health Organization.
P. aeruginosa is commonly found in the environment, including in soil and freshwater. Reservoirs in hospital settings can include drains, taps, sinks and equipment washers.
While the bacterium generally does not affect healthy people it can cause infections in individuals with cystic fibrosis and those who are immunocompromised, such as patients with burns, cancer and many other serious conditions. Patients with invasive devices such as catheters are also at risk due to the ability of P. aeruginosa to form biofilms on the surfaces of these devices.
P. aeruginosa, like other gram-negative bacteria, is enclosed in a second, fatty outer membrane that acts as a protective capsule, making it more difficult to kill.
How QACs kill
QACs have a nitrogen atom at the center of four carbon chains. In simplest terms, the positively charged head of the nitrogen center is drawn to the negatively charged phosphates of the fatty acids encasing P. aeruginosa and many other bacteria and viruses. The heads of the carbon chains act like spearpoints, stabbing into both protective fatty membranes and inner cellular membranes and causing pathogens to disintegrate.
The researchers tested 20 different drug-resistant strains of P. aeruginosa collected from hospitals around the world by the Walter Reed National Military Medical Center as part of the Multidrug-Resistant Organism Repository and Surveillance Network.
The results showed that all 20 strains were at least partially resistant to QACs — the common active ingredient in most front-line cleaning agents — and 80% of the strains were fully resistant to QACs.
“This mechanism has worked for 100 years essentially by slicing into the outer and inner membranes of a pathogen and destroying them,” Wuest says. “We were surprised to see the level at which that appears to no longer be the case.”
Improper use of cleaning agents may be one factor leading to resistance, Wuest theorizes.
“QACs don’t immediately kill,” he explains. “After application, it’s important to wait four or five minutes before wiping these cleaning agents away. It’s also important to use the right concentration. If used inappropriately, some bacteria can survive, which can lead to them developing resistance.”
Greater use of cleaning agents during the COVID-19 pandemic may have given P. aeruginosa and some other hard-to-kill pathogens more opportunities to develop resistance, he adds.
A new method that ‘works surprisingly well’
For the current paper, the researchers also tested the resistance of the panel of multidrug-resistant P. aeruginosa strains against a new quaternary phosphonium compound, or QPC, developed in the Wuest and Minbiole labs. The results showed that the compound was highly effective at killing all 20 of the resistant P. aeruginosa strains.
“It works surprisingly well even at a low concentration,” Vargas-Cuebas says.
The researchers demonstrated that their novel QPC works not by piercing the protective outer capsule of a P. aeruginosa bacterium but by diffusing through this outer membrane and then selectively attacking the inner cellular membrane.
“It’s counterintuitive,” Wuest remarks. “You would think that the approach of conventional biocides, to take out both membranes, would be a more effective way to kill P. aeruginosa. Why does passively diffusing through the outer membrane and focusing on attacking the inner membrane make our QPC compound more effective? We don’t know yet. It’s like a magic trick.”
They showed that this same mechanism underlies the effectiveness of two commercial antiseptics: octenidine, more commonly used in Europe as a hospital antiseptic, and chlorhexidine, a common ingredient in mouthwashes.
Wuest and colleagues plan to continue research into how this newly identified mechanism may work against an array of pathogens and how that might translate into new biocides and more effective cleaning protocols in hospitals and other settings.
“Our work is paving the way for much-needed innovations in disinfectant research,” Wuest says.
Source: Emory University