Bayer Issues Recall on YAZ Plus Contraceptive Pills
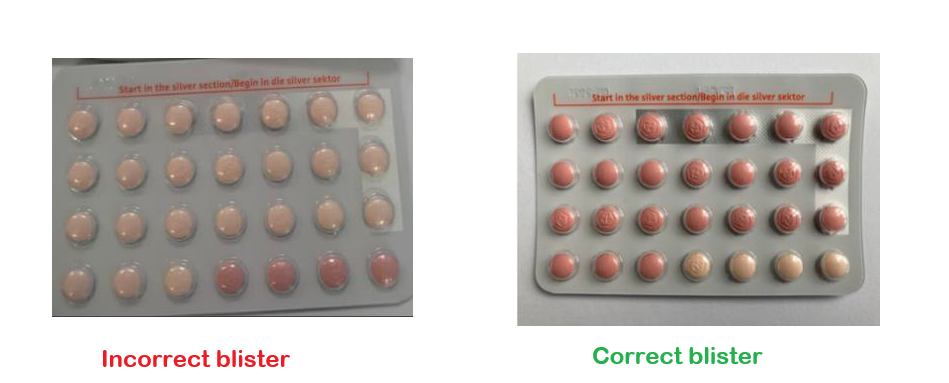
On November 21, Bayer (Pty) Ltd issued a medicine recall for a specific batch (WEW96J) of YAZ PLUS tablets. In a press release, they explain the reason for the recall: it has been discovered that the active and inactive tablets in this batch are swapped. This mix-up has resulted in some packs containing only four hormone tablets instead of the required 24, and 24 hormone-free tablets instead of four, compromising the product’s contraceptive efficacy.
The company advises that healthcare professionals, wholesalers, hospitals, retail pharmacy outlets, doctors, nurses, pharmacists, authorised prescribers, dispensers, and individual customers or patients in possession of the affected batch can return product to their healthcare facility from which it was dispensed, for credit.
Bayer urges that if you are in possession of YAZ PLUS tablets from the affected batch, to do the following:
- Stop Use Immediately: If you have been taking the tablets from a batch that is affected with the mix-
up, stop taking them immediately and contact your healthcare professional. While only a limited number of packs from the respective batch is affected, as a precautionary measure, no tablets from these packs shall be used until you have consulted your Healthcare Practitioner, as they may potentially not provide the contraceptive protection you expect. - Return the Product: Please return any affected packs to the pharmacy or retailer where you
purchased them for a replacement or refund. - Check Your Packs: If you have multiple packs of YAZ PLUS, please check each one of them, to
ensure they are not from the affected batch. - Consult Healthcare Provider: If you have consumed tablets from the affected batch, or if you have
concerns about your contraceptive coverage, please consult your healthcare provider as soon as
possible for advice.
In the press release, Bayer says that it “takes the safety and efficacy of its products seriously and is committed to ensuring that all YAZ PLUS tablets in the market meet the highest quality standards.” It further advises that the root cause for the mix-up of tablets in the packaging has been identified and corrective measures taken. Only this one batch – and no others – was affected.
“The company is working diligently with SAHPRA and healthcare providers to facilitate the recall process and minimise any inconvenience to our customers. We are dedicated to addressing this issue promptly and ensuring the continued health and safety of all our customers.”
Further Information and Support:
For more information about this recall, or if you have any questions or concerns, please contact Bayer +27
(0) 11 921 5000. Our team is available to provide the support and information you need.
Report a side effect: Patient Safety Reporting – Introduction
Report a product quality complaint for Pharmaceutical Products: afptc@bayer.com



