An Inhalable Chemotherapy for Lung Cancer – Inspired by Mussels
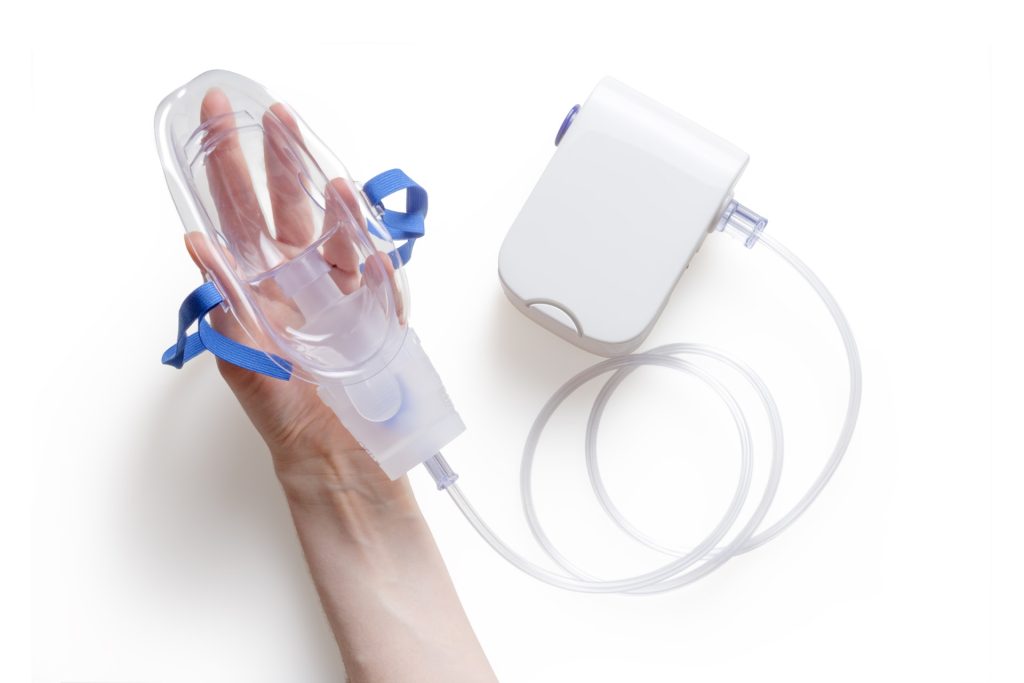
Researchers from POSTECH and Kyungpook National University have developed a novel inhalable therapeutic delivery system for lung cancer, making use of mucoadhesive protein nanoparticles inspired the adhesive properties of marine mussels.
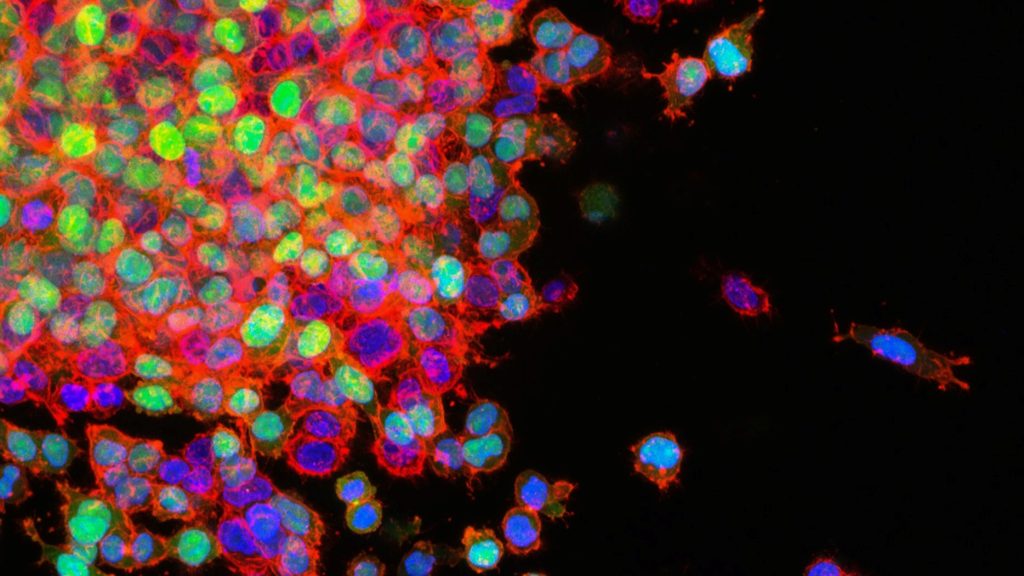
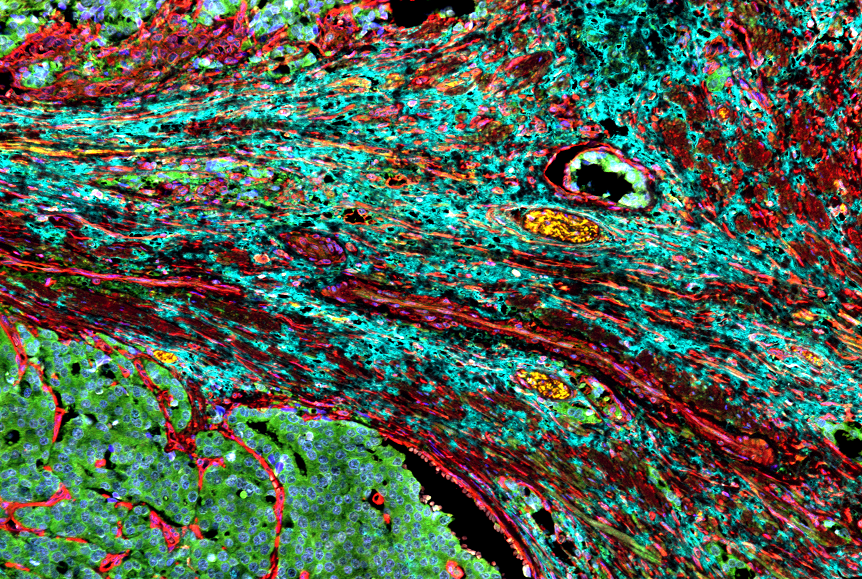
Non-small cell lung cancer (NSCLC), which accounts for 85% of all lung cancer cases and treatment is particularly challenging due to difficulties in early detection. Current anticancer treatments are predominantly administered intravenously, impacting both malignant and healthy tissues, often leading to severe adverse effects. As a result, inhalable therapeutics have emerged as a promising alternative, enabling localised drug delivery directly to the lungs. A major obstacle to this approach is the lung’s mucosal barriers and immune cells. Building on this context, collaborative research has culminated in the development of a mucoadhesive protein nanoparticle designed for lung cancer treatment.
This approach leverages the remarkable adhesive properties of marine mussel proteins, renowned for their underwater adhesion. Drawing inspiration from the oxidation-reduction mechanisms of foot protein type 6 (fp-6), the researchers engineered foot protein type 1 (fp-1) by integrating cysteine, creating a biomaterial with enhanced adhesive strength and precise drug delivery capabilities within the lung cancer microenvironment. These nanoparticles exhibit exceptional therapeutic efficacy by enabling selective payload release while effectively inhibiting release in healthy tissues to minimise adverse effects. Moreover, the intrinsic biocompatibility, biodegradability, and immunocompatibility of marine mussel proteins ensure superior biological safety and substantially prolong the retention of anticancer drugs, thereby amplifying their therapeutic impact.
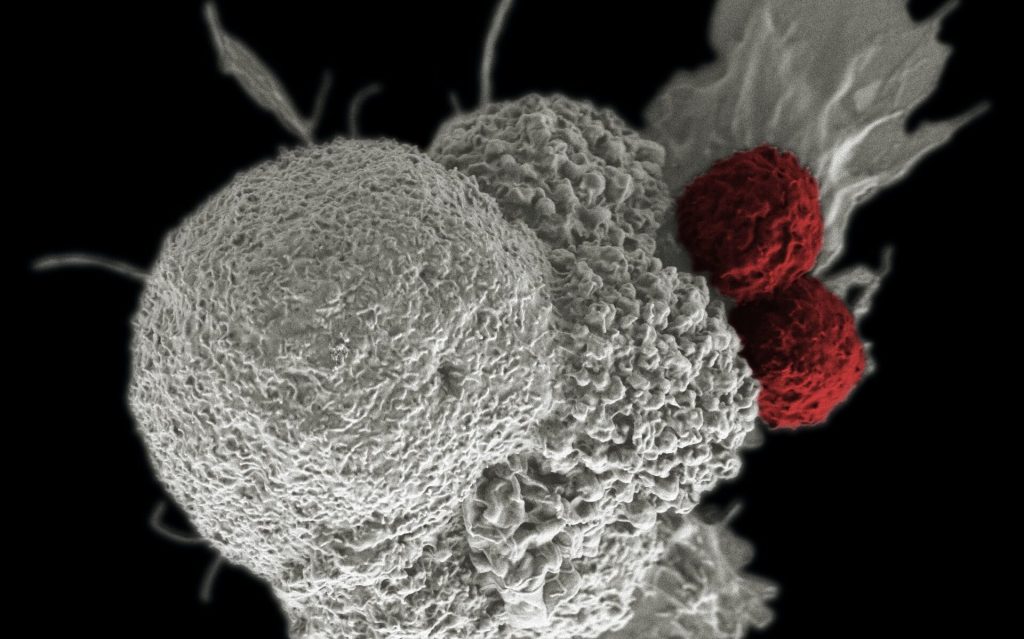
In animal models of lung cancer, the nanoparticles developed by the research team and their contained anti-cancer drugs showed effectiveness in inhibiting cancer cell metastasis and invasion after being delivered to the lungs through a nebuliser and adhering to the mucosa for extended periods. This advancement holds the potential to enhance patient access to lung cancer treatment, as the simplified inhalation-based drug administration could be self-managed at home. Furthermore, this approach may significantly improve patients’ quality of life by reducing the need for hospital visits.
Professor Hyung Joon Cha, who spearheaded the collaborative research at POSTECH, stated, “The findings from our study have the potential to substantially enhance both the precision and efficacy of lung cancer treatments, while significantly improving patients’ quality of life.”