Radiopharmaceuticals Being Tested for Brain Tumours in Children
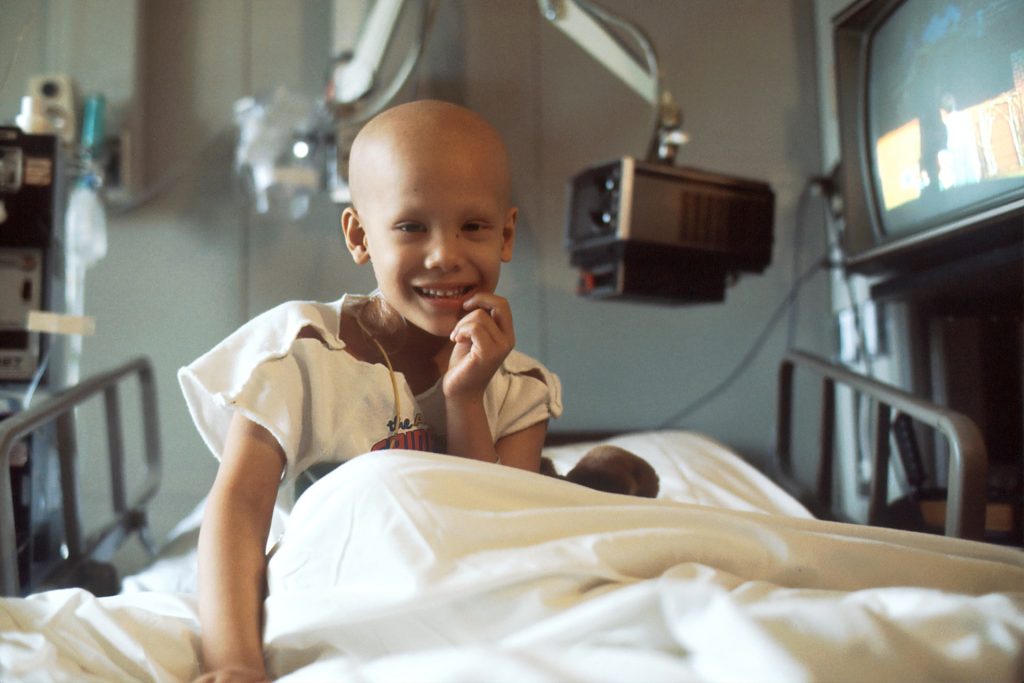
Neuroblastoma is a rare disease that affects children, often before the age of two. Some are born with the disease. Paediatric surgeon Jakob Stenman is investigating whether targeted radioactive drugs can slow down the disease in those with the most severe form.
Neuroblastoma is a complicated disease, with the most aggressive variant called high-risk neuroblastoma. Children with this disease are treated very intensively. They may undergo surgery, chemotherapy, high-dose chemotherapy with stem cell transplantation, radiotherapy and antibody treatment. Treatment often lasts up to a year and a half.
Despite this, the survival rate is around 60%, according to the Swedish Childhood Cancer Foundation.
“Some relapse in their disease, and we currently lack curative treatment for them,” says Jakob Stenman, a researcher at the Department of Women’s and Children’s Health at Karolinska Institutet.
It is these children, those who have relapsed, that he is treating in a study with targeted radioactive drugs. These are molecules that attach to the surface of cancer cells. These molecules have an appendage: the radioactive substance lutetium-177. The drug first moves through the bloodstream but then attaches to the cancer cells. The emitted radiation damages the cancer cells but unfortunately also the neighbouring healthy cells.
“We have treated ten children so far. Unfortunately, the disease has not disappeared in any of these cases, but it seems to be slowing down, and some benefit more than others from the treatment. When it comes to side effects, the children have tolerated the treatment well,” says Jakob Stenman.
The hope is to be able to prevent relapse
He reports that the interest has been great from clinics in other countries where these children are treated. Hospitals from Lithuania, the Netherlands, the United Kingdom and are now involved.
In neuroblastoma, cancer cells often look very different, even in the same patient. In some metastases, there may be many cells with a surface where the drug attaches, while in other metastases there may be fewer such cells. This means that the targeted drug attaches to fewer cells in some of the metastases. As a result, the local radiation dose is too low in these metastases, which can then continue to grow and spread further.
Jakob Stenman therefore believes that the treatment could be more effective if the radioactive substance used is even more potent, which in this context means that it emits even more energy (ie, radiation). If it then attaches to fewer cells in a metastasis, it might still be able to eliminate all the cancer cells there. But it must act even more locally to protect other tissues from the higher radiation dose.
The researchers have identified several substances they believe could work in this way. These include actinium-225, astatine-211 or lead-212. The effects and side effects of actinium-225 are now being investigated in cell studies and animal experiments. The goal is to start a clinical trial with actinium in three to five years.
“If what we believe turns out to be true, we hope to be able to prevent relapse and thereby enable a cure for a larger proportion of children who have developed high-risk neuroblastoma,” says Jakob Stenman.
Text: Annika Lund for Medicinsk Vetenskap nr 4 2024
Source: Karolinska Institutet