Goldeneye: Research on Restoring Eyesight with Gold Nanoparticles
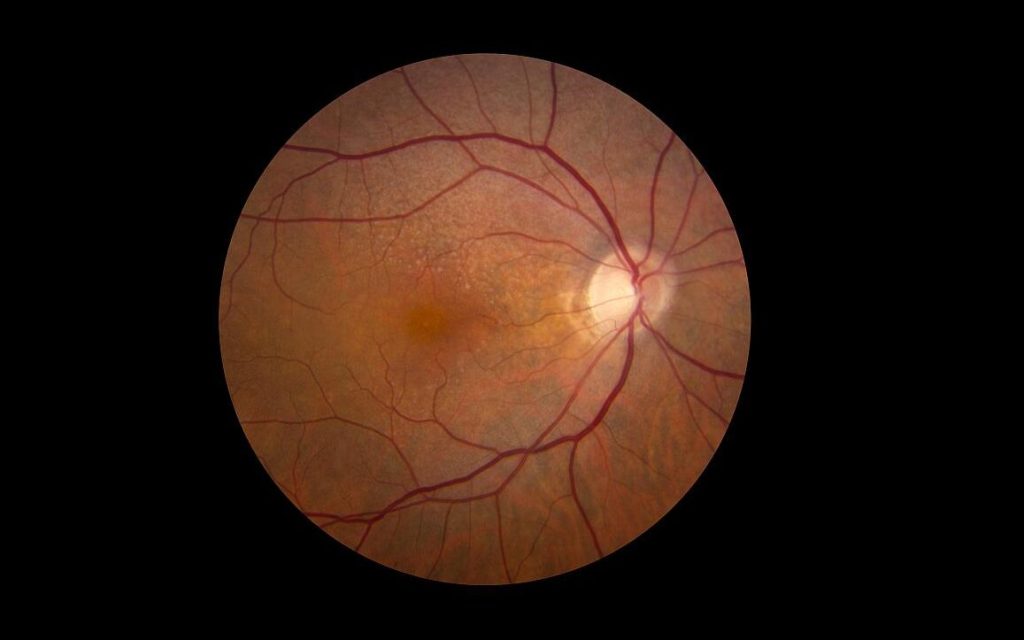
A new study by Brown University researchers suggests that gold nanoparticles might one day be used to help restore vision in people with macular degeneration and other retinal disorders.
In a study published in the journal ACS Nano and supported by the National Institutes of Health, the research team showed that nanoparticles injected into the retina can successfully stimulate the visual system and restore vision in mice with retinal disorders. The findings suggest that a new type of visual prosthesis system in which nanoparticles, used in combination with a small laser device worn in a pair of glasses or goggles, might one day help people with retinal disorders to see again.
“This is a new type of retinal prosthesis that has the potential to restore vision lost to retinal degeneration without requiring any kind of complicated surgery or genetic modification,” said Jiarui Nie, research leader and now a postdoctoral researcher. “We believe this technique could potentially transform treatment paradigms for retinal degenerative conditions.”
Nie performed the work while working in the lab of Jonghwan Lee, an associate professor in Brown’s School of Engineering and a faculty affiliate at Brown’s Carney Institute for Brain Science, who oversaw the work and served as the study’s senior author.
Retinal disorders like macular degeneration and retinitis pigmentosa affect millions of people in the U.S. and around the world. These conditions damage light-sensitive cells in the retina called photoreceptors — the “rods” and “cones” that convert light into tiny electric pulses. Those pulses stimulate other types of cells further up the visual chain called bipolar and ganglion cells, which process the photoreceptor signals and send them along to the brain.
This new approach uses nanoparticles injected directly into the retina to bypass damaged photoreceptors. When infrared light is focused on the nanoparticles, they generate a tiny amount of heat that activates bipolar and ganglion cells in much the same way that photoreceptor pulses do. Because disorders like macular degeneration affect mostly photoreceptors while leaving bipolar and ganglion cells intact, the strategy has the potential to restore lost vision.
In this new study, the research team tested the nanoparticle approach in mouse retinas and in living mice with retinal disorders. After injecting a liquid nanoparticle solution, the researchers used patterned near-infrared laser light to project shapes onto the retinas. Using a calcium signal to detect cellular activity, the team confirmed that the nanoparticles were exciting bipolar and ganglion cells in patterns matched the shapes projected by the laser.
The experiments showed that neither the nanoparticle solution nor the laser stimulation caused detectable adverse side effects, as indicated by metabolic markers for inflammation and toxicity. Using probes, the researchers confirmed that laser stimulation of the nanoparticles caused increased activity in the visual cortices of the mice — an indication that previously absent visual signals were being transmitted and processed by the brain. That, the researchers say, is a sign that vision had been at least partially restored, a good sign for potentially translating a similar technology to humans.
For human use, the researchers envision a system that combines the nanoparticles with a laser system mounted in a pair of glasses or goggles. Cameras in the goggles would gather image data from the outside world and use it to drive the patterning of an infrared laser. The laser pulses would then stimulate the nanoparticles in people’s retinas, enabling them to see.
The approach is similar to one that was approved by the Food and Drug Administration for human use a few years ago. The older approach combined a camera system with a small electrode array that was surgically implanted in the eye. The nanoparticle approach has several key advantages, according to Nie.
For starters, it’s far less invasive. As opposed to surgery, “an intravitreal injection is one of the simplest procedures in ophthalmology,” Nie said.
There are functional advantages as well. The resolution of the previous approach was limited by the size of the electrode array — about 60 square pixels. Because the nanoparticle solution covers the whole retina, the new approach could potentially cover someone’s full field of vision. And because the nanoparticles respond to near-infrared light as opposed to visual light, the system doesn’t necessarily interfere with any residual vision a person may retain.
More work needs to be done before the approach can be tried in a clinical setting, Nie said, but this early research suggests that it’s possible.
“We showed that the nanoparticles can stay in the retina for months with no major toxicity,” Nie said of the research. “And we showed that they can successfully stimulate the visual system. That’s very encouraging for future applications.”
Source: Brown University