World’s First Gene-edited Pig Kidney Transplant into Living Recipient
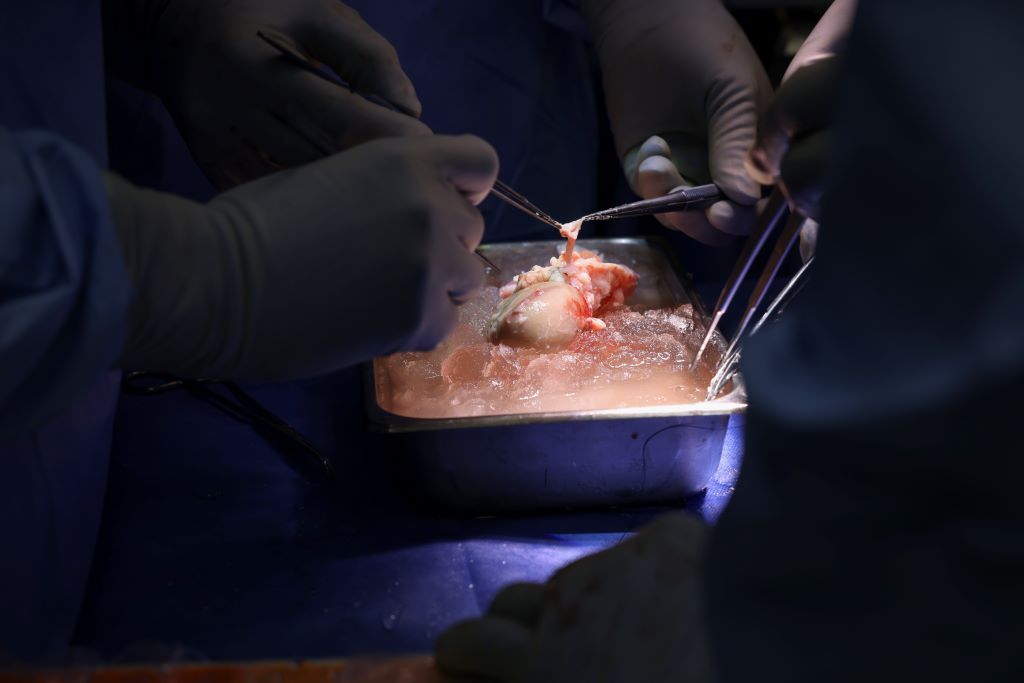
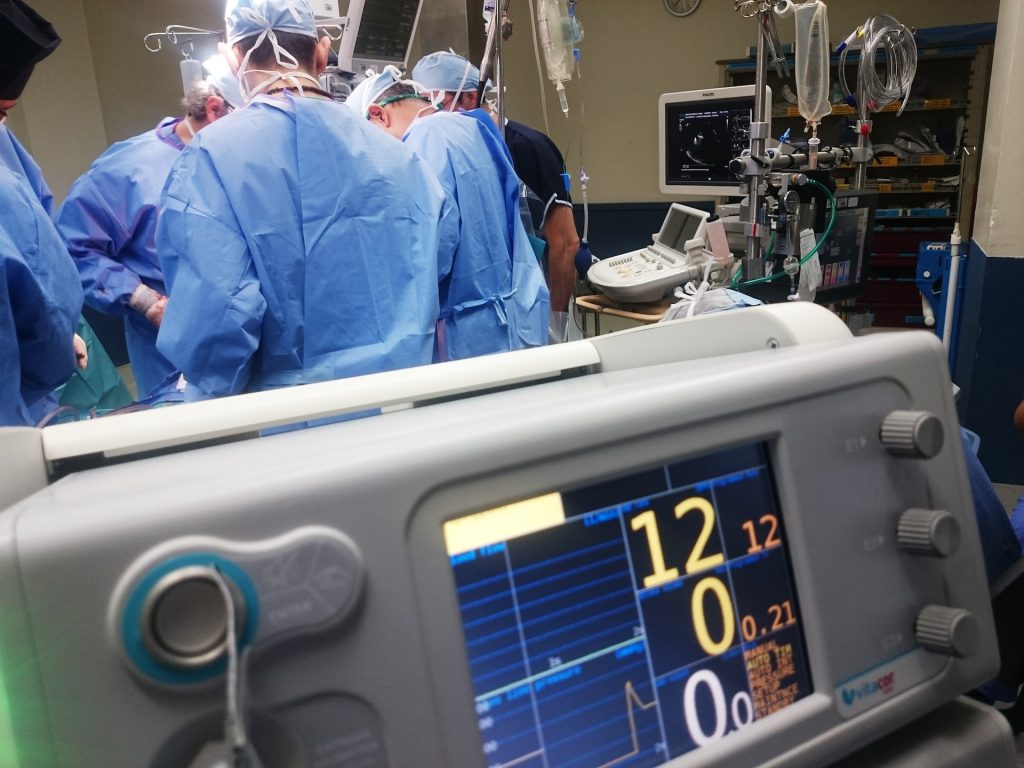
Massachusetts General Hospital (MGH) has announced the world’s first successful transplant of a genetically-edited pig porcine) kidney into a 62-year-old man living with end-stage kidney disease (ESKD). Surgeons from the Mass General Transplant Center conducted the four-hour-long surgery on Saturday, March 16. The procedure marks a major milestone in the quest to provide more readily available organs to patients. Mass General Brigham is an internationally recognised leader in transplantation services, providing advanced care for a wide spectrum of organ and tissue transplants throughout its renowned academic medical system.
Under the leadership of Leonardo V. Riella, MD, PhD, Medical Director for Kidney Transplantation, Tatsuo Kawai, MD, PhD, Director of the Legorreta Center for Clinical Transplant Tolerance, along with Nahel Elias, MD, Interim Chief of Transplant Surgery and Surgical Director for Kidney Transplantation, a genetically-edited pig kidney with 69 genomic edits was successfully transplanted into a living patient.
Mass General Brigham has a rich history in organ transplant innovation, including the world’s first successful human organ transplant (kidney) performed at Brigham and Women’s Hospital in 1954 and the nation’s first penile transplant, performed at MGH in 2016. Mass General Brigham transplantation programs draw upon the deep, integrated expertise of some of the world’s leading transplant physicians and scientists who collaborate across experienced multidisciplinary teams to advance medicine and improve the lives of patients.
“Mass General Brigham researchers and clinicians are constantly pushing the boundaries of science to transform medicine and solve significant health issues facing our patients in their daily lives,” said Anne Klibanski, MD, President and CEO, Mass General Brigham. “Nearly seven decades after the first successful kidney transplant, our clinicians have once again demonstrated our commitment to provide innovative treatments and help ease the burden of disease for our patients and others around the world.”
“The tireless commitment of our clinicians, researchers and scientists to improving the lives of our transplant patients – both current and future – is at the very heart and soul of academic medicine and what it means to work and provide care at Mass General Brigham,” said David F. M. Brown, MD, President, Academic Medical Centers, Mass General Brigham. “We are so thankful to the incredible staff throughout our hospitals who helped make this surgery a success, and to the patient for his bravery and courage.”
“The success of this transplant is the culmination of efforts by thousands of scientists and physicians over several decades. We are privileged to have played a significant role in this milestone. Our hope is that this transplant approach will offer a lifeline to millions of patients worldwide who are suffering from kidney failure,” Kawai said.
The pig kidney was provided by eGenesis, from a pig donor that was genetically-edited using CRISPR-Cas9 technology to remove harmful pig genes and add certain human genes to improve its compatibility with humans. Additionally, scientists inactivated porcine endogenous retroviruses in the pig donor to eliminate any risk of infection in humans. Over the past five years, MGH and eGenesis have conducted extensive collaborative research, with the findings published in Nature in 2023.
This successful procedure in a living recipient is a historic milestone in the emerging field of xenotransplantation – the transplantation of organs or tissues from one species to another – as a potential solution to the worldwide organ shortage. According to the United Network for Organ Sharing (UNOS), more than 100 000 people in the U.S. await an organ for transplant and 17 people die each day waiting for an organ. A kidney is the most common organ needed for transplant, and end-stage kidney disease rates are estimated to increase 29-68% in the U.S. by 2030, according to literature published in the Journal of the American Society of Nephrology.
The patient, Mr. Richard ‘Rick’ Slayman of Weymouth, Mass., is recovering well at MGH and is expected to be discharged soon.
“The real hero today is the patient, Mr Slayman, as the success of this pioneering surgery, once deemed unimaginable, would not have been possible without his courage and willingness to embark on a journey into uncharted medical territory. As the global medical community celebrates this monumental achievement, Mr Slayman becomes a beacon of hope for countless individuals suffering from end-stage renal disease and opens a new frontier in organ transplantation,” said Joren C. Madsen, MD, DPhil, Director of the MGH Transplant Center.
Mr Slayman said in a statement, “I have been a Mass General Transplant Center patient for 11 years and have the highest level of trust in the doctors, nurses, and clinical staff who have cared for me. When my transplanted kidney began failing in 2023, I again trusted my care team at MGH to meet my goals of not just improving my quality of life but extending it. My nephrologist, Dr Winfred Williams, MD and the Transplant Center team suggested a pig kidney transplant, carefully explaining the pros and cons of this procedure. I saw it not only as a way to help me, but a way to provide hope for the thousands of people who need a transplant to survive. I want to thank everyone at MGH who has cared for me, especially Dr Williams, Dr Kawai, the surgeon who performed my first kidney transplant and now this one, and Dr Riella, who has orchestrated the logistics behind this new transplant. They have supported me during every step of the journey, and I have faith they will continue to do so.”
Mr Slayman, who has been living with Type 2 diabetes and hypertension for many years, previously received a kidney transplant from a human deceased donor in December 2018, performed at MGH by Kawai, after being on dialysis seven years prior. The transplanted kidney showed signs of failure approximately five years later and Mr Slayman resumed dialysis in May 2023. Since resuming dialysis, he encountered recurrent dialysis vascular access complications requiring visits to the hospital every two weeks for de-clotting and surgical revisions, significantly impacting his quality of life and a common problem among dialysis patients.
“The continued success of this groundbreaking kidney transplant represents a true milestone in the field of transplantation. It also represents a potential breakthrough in solving one of the more intractable problems in our field, that being unequal access for ethnic minority patients to the opportunity for kidney transplants due to the extreme donor organ shortage and other system-based barriers. This health disparity has been the target of many national policy initiatives for over 30 years, with only limited success. An abundant supply of organs resulting from this technological advance may go far to finally achieve health equity and offer the best solution to kidney failure – a well-functioning kidney – to all patients in need. I commend Mr Slayman, who has been my patient for many years, for his courageousness in becoming a trailblazer in the field of transplantation,” Williams said.
The procedure was performed under a single FDA Expanded Access Protocol (EAP) – known as compassionate use – granted to a single patient or group of patients with serious, life-threatening illnesses or conditions to gain access to experimental treatments or trials when no comparable treatment options or therapies exist. Mr. Slayman also received infusion of novel immunosuppressant drugs, tegoprubart, provided by Eledon Pharmaceuticals, Inc., and ravulizumab, provided by Alexion Pharmaceuticals, Inc.
Source: Massachusetts General Hospital