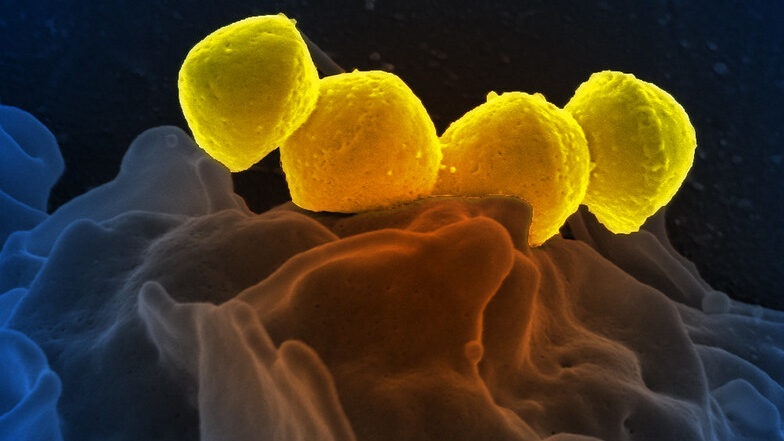
Research Shines a Light on Emerging Virulent Streptococcus Subspecies
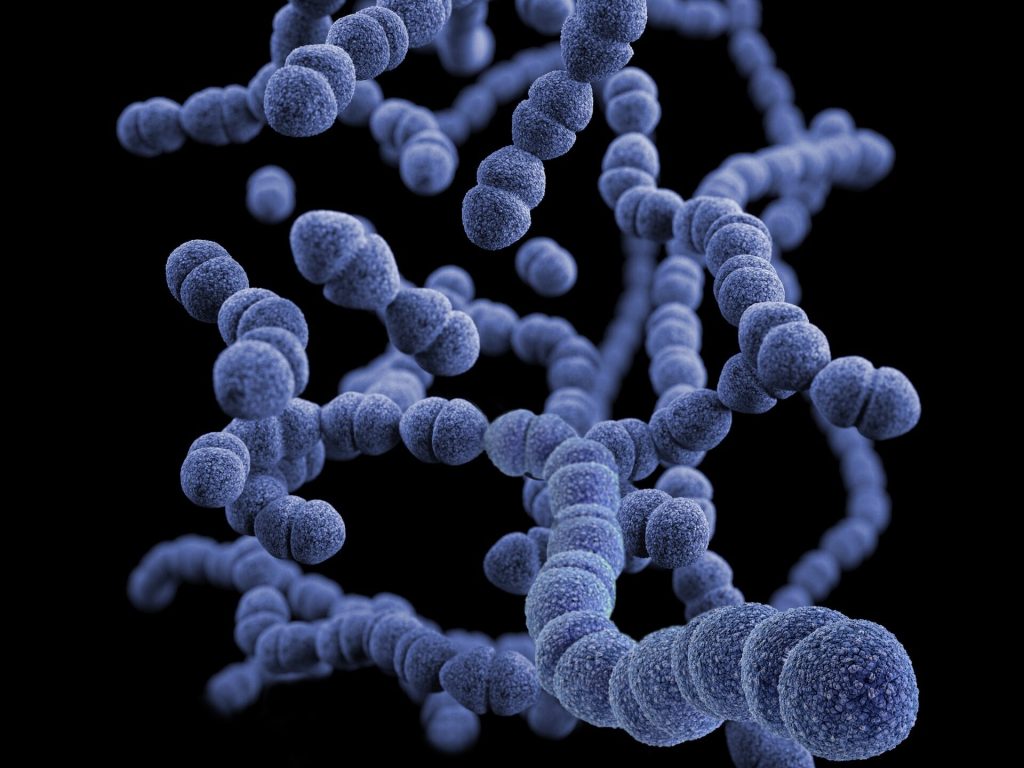
A concerning increase in global rates of severe invasive infections becoming resistant to key antibiotics has a team of infectious disease researchers at the Houston Methodist Research Institute studying a recently emerged strain of bacteria, Streptococcus dysgalactiae subspecies equisimilis (SDSE). SDSE infects humans via the skin, throat, gastrointestinal tract and female genital tract to cause infections ranging in severity from pharyngitis to necrotising fasciitis. The findings of this study are described in a paper appearing in the journal mBio.
Though closely related to group A streptococcus (also commonly known as Streptococcus pyogenes), which has been very well studied, little is known about SDSE.
“Given its great emerging importance to human health, our limited understanding of SDSE molecular pathogenesis is remarkable,” said Jesus M. Eraso, PhD, an assistant research professor of pathology & genomic medicine with Houston Methodist and lead author on the study.
To close this knowledge gap, the Houston Methodist team used a sophisticated integrative approach to study 120 human isolates of a particular SDSE subtype, called stG62647. They analysed the subtype’s genome, where the information of its DNA is stored, its transcriptome, which provides a snapshot of the complete gene expression profile at the time the SDSE cells were collected, and its virulence, which refers to the degree of damage it causes to its host. The stG62647 SDSE strains are important to study because they have been reported to cause unusually severe infections, and understanding the relationships and interplay between these three entities gave the researchers a richer understanding of how it causes disease.
The data from this integrative analysis provided much new data about this important emerging human bacterial pathogen and are useful in vaccine research. It also raised many new questions and generated new hypotheses to be studied in this ongoing line of investigation.