Immunotherapy Blocks Scarring, Improves Cardiac Function in Heart Failure
A new study from Washington University School of Medicine in St. Louis suggests that a type of immunotherapy also may be an effective treatment strategy for heart failure by using an FDA-approved drug to block the signalling protein IL-1 beta. The study is published in Nature.
After a heart attack, viral infection or other injury to the heart, scar tissue often forms in the heart muscle, where it interferes with the heart’s normal contractions and plays a leading role in heart failure, a chronic condition which can only be slowed, not cured.
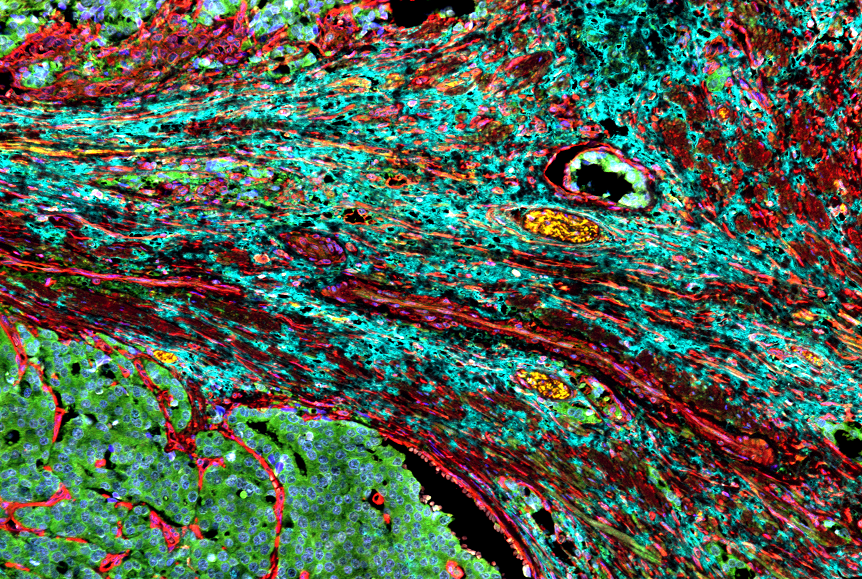
Studying human tissue samples as part of the new study, the researchers identified a type of fibroblast cell in the heart as the main culprit responsible for the formation of scar tissue in heart failure. To see if they could prevent scar formation, the scientists turned to mouse models of heart failure that have the very same type of fibroblasts. They used a therapeutic monoclonal antibody that blocks the formation of this harmful type of fibroblast, and succeeded in reducing the formation of scar tissue and improving heart function in the mice.
“After scar tissue forms in the heart, its ability to recover is dramatically impaired or impossible,” said cardiologist and senior author Kory Lavine, MD, PhD, a professor of medicine in the Cardiovascular Division at WashU Medicine. “Heart failure is a growing problem in the US and globally, affecting millions of people. Current treatments can help relieve symptoms and slow the progression, but there is a tremendous need for better therapies that actually stop the disease process and prevent the formation of new scar tissue that causes a loss of heart function. We are hopeful our study will lead to clinical trials investigating this immunotherapy strategy in heart failure patients.”
Fibroblasts have many roles in the heart, and parsing out the differences between various populations of these cells has been challenging. Some types of fibroblasts support the heart’s structural integrity and maintain good blood flow through the heart’s blood vessels, while others are responsible for driving inflammation and the development of scar tissue. Only recently, with the wide availability of the most advanced single cell sequencing technologies, could scientists peg which groups of cells are which.
“These various types of fibroblasts highlight newly recognised opportunities to craft treatment strategies that specifically block the type of fibroblasts that promote scarring and protect fibroblasts that maintain the structure of the heart, so the heart doesn’t rupture,” Lavine said. “Our research suggests that the fibroblasts that promote scarring in the injured heart are very similar to fibroblasts associated with cancer and other inflammatory processes. This opens the door to immunotherapies that potentially can stop the inflammation and resulting scar tissue.”
The research team, co-led by Junedh Amrute, a graduate student in Lavine’s lab, used genetic methods to demonstrate that a signaling molecule called IL-1 beta was important in a chain of events driving fibroblasts to create scar tissue in heart failure. With that in mind, they tested a mouse monoclonal antibody that blocks IL-1 beta and found beneficial effects in the mouse hearts. The mouse monoclonal antibody was provided by Amgen, whose scientists were also co-authors of the study. Monoclonal antibodies are proteins manufactured in the lab that modulate the immune system. The treatment reduced the formation of scar tissue and improved the pumping capacity of the mouse hearts, as measured on an echocardiogram.
At least two FDA-approved monoclonal antibodies, canakinumab and rilonacept, can block IL-1 signalling. These immunotherapies are approved to treat inflammatory disorders such as juvenile idiopathic arthritis and recurrent pericarditis, which is inflammation of the sac surrounding the heart.
One of these antibodies also has been evaluated in a clinical trial for atherosclerosis, a buildup of plaque that hardens the arteries. The trial, called CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study), showed a benefit for study participants with atherosclerosis.
“Even though this trial was not designed to test this treatment in heart failure, there are hints in the data that the monoclonal antibody might be beneficial for patients with heart failure,” Lavine said. “Secondary analyses of the data from this trial showed that the treatment was associated with a sizable reduction in heart failure admissions compared with standard care. Our new study may help explain why.”
Even so, the IL-1 antibody used in the CANTOS study had some side effects, such as increased risk of infection, that could perhaps be reduced with a more targeted antibody that specifically blocks IL-1 signaling in cardiac fibroblasts, according to the researchers.
“We are hopeful that the combination of all of this evidence, including our work on the IL-1 beta pathway, will lead to the design of a clinical trial to specifically test the role of targeted immunotherapy in heart failure patients,” Lavine said.