Don’t Overlook Latent Autoimmune Diabetes in Adults, Researchers Caution
To reduce the risk of complications, it is important to measure antibodies those with adult onset diabetes, while also considering the levels of these antibodies.
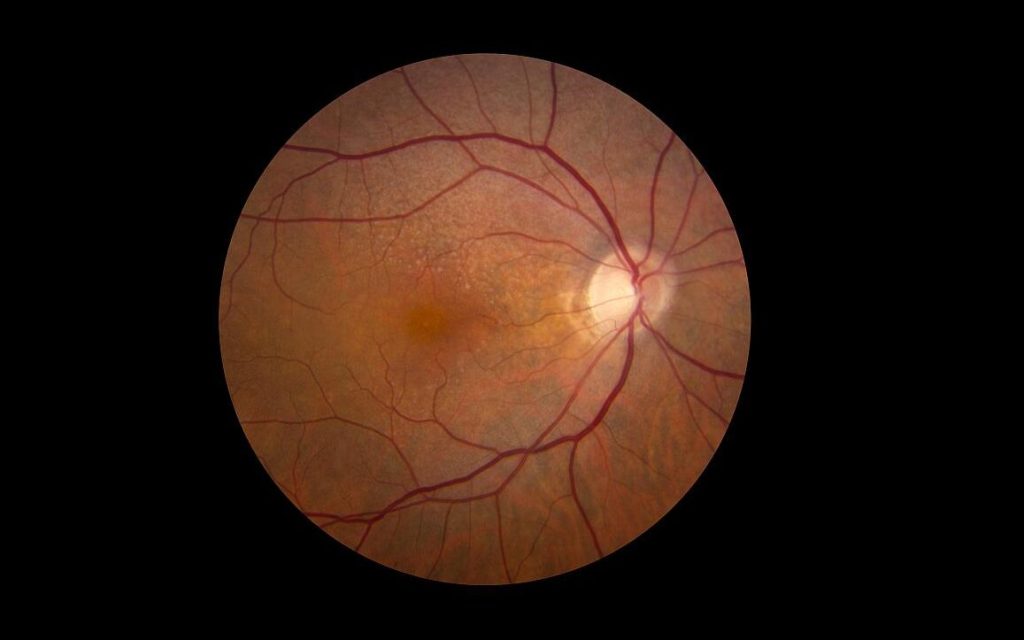
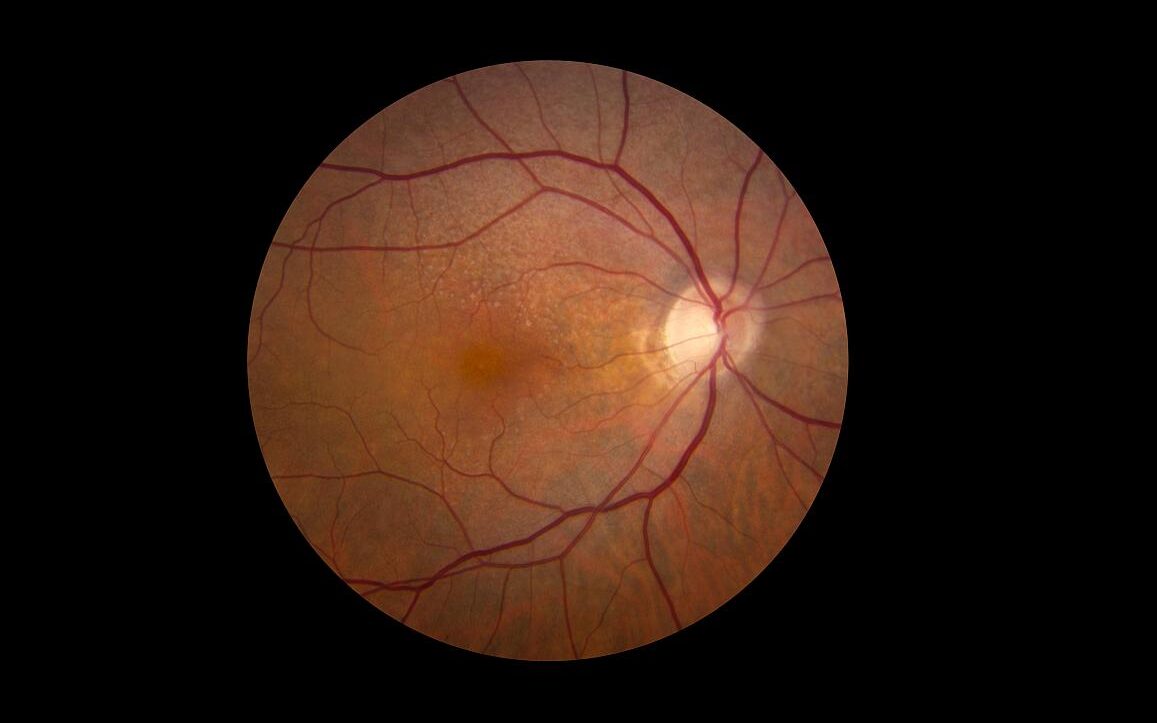
In a study published in the journal Diabetes Care, researchers demonstrate that individuals with Latent Autoimmune Diabetes in Adults (LADA) have an equally high risk of developing cardiovascular disease as people with type 2 diabetes, but a higher risk of developing retinopathy and poorer glucose control. Many also lack adequate treatment.
LADA is a common but relatively unknown form of diabetes. Similar to type 1 diabetes, it is an autoimmune disease characterised by antibodies against insulin-producing cells. It develops in adulthood, and the autoimmune process progresses more slowly than in type 1 diabetes. LADA also shares features with type 2 diabetes, which means those affected risk getting the wrong diagnosis if antibodies are not measured. Incorrect diagnosis can result in inadequate treatment. Previous studies suggest that between five and ten percent of all individuals initially diagnosed with type 2 diabetes actually have LADA. Researchers at Karolinska Institutet, and the Universities of Lund and Helsinki set out to examine the risk of complications in LADA.
Our results emphasise the importance of diagnosing LADA correctly and careful monitoring of glucose control in these individuals, so that treatment can be intensified if needed, thereby reducing the risk of complications.
Yuxia Wei, PhD-student and Sofia Carlsson, senior lecturer, Institute of Environmental Medicine, Karolinska Institutet
According to the study LADA was characterised by fewer metabolic risk factors than type 2 diabetes, such as high blood pressure and high blood lipids. However, a lower proportion of individuals with LADA achieved good glucose control. The lack of glucose control was most evident in LADA patients with high levels of the antibody GADA (glutamic acid decarboxylase antibody). A significant portion of individuals with LADA lacked any glucose-lowering treatment.
The results of the new study are based on the ESTRID study, where researchers followed over 4000 individuals with diabetes, of whom 550 had LADA, for up to 12 years after diagnosis. According to the researchers, it is the most comprehensive study to date regarding the risk of complications in LADA.
Source: Karolinska Institutet