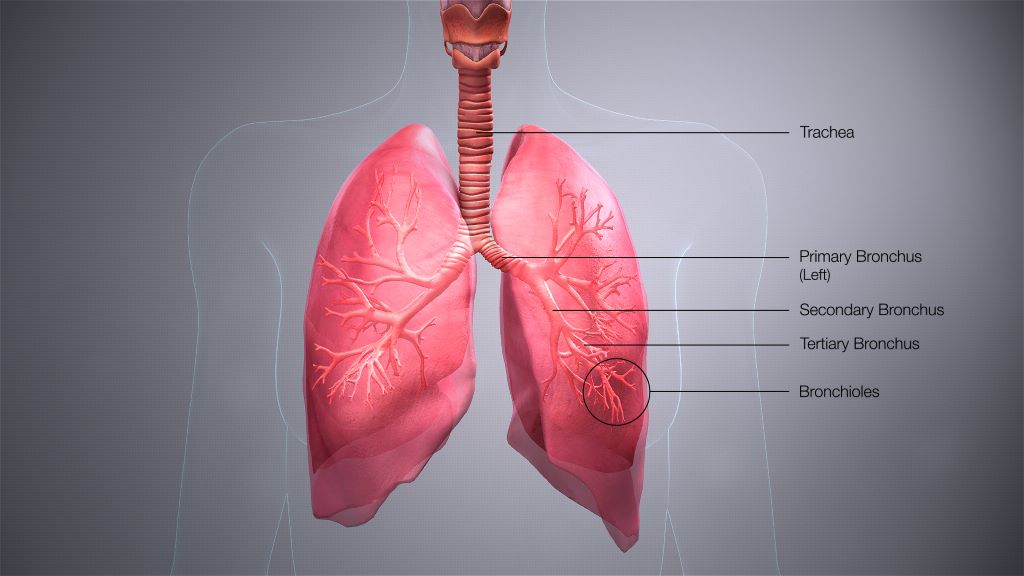
Time is Running out to Develop a Paxlovid Alternative
Researchers from Rutgers University in the U.S. believe that they are ahead in a race to find an oral COVID-19 treatment to supplement or replace the antiviral Paxlovid. Their report, published in Science, shows that an alternative medication, a viral papain-like protease inhibitor, inhibits disease progression in animals while also possessing an important advantage over Paxlovid – fewer prescription drug contraindications.
“COVID-19 remains the nation’s third leading cause of death, so there’s already a massive need for additional treatment options,” said Jun Wang, senior author of the study and associate professor at Rutgers. “That need will grow more urgent when, inevitably, COVID-19 mutates in ways that prevent Paxlovid from working.”
The Rutgers team hoped to make a drug that interfered with viral papain-like protease (PLpro), a protein that performs important functions in all known strains of COVID-19.
Creating such a drug required detailed information about PLpro’s structure, which Wang’s team got from the Arnold Lab at Rutgers’ Center for Advanced Biotechnology and Medicine (CABM).
Precise knowledge of PLpro’s structure enabled Wang’s team to design and synthesise 85 drug candidates that would bond to – and interfere with – this vital protein.
“The PLpro crystal structures showed an unexpected arrangement of how the drug candidate molecules bind to its protein target, leading to innovative design ideas implemented by professor Wang’s medicinal chemistry team,” said Eddy Arnold, who is a professor at CABM.
Laboratory testing established that the most effective of those drug candidates, a compound dubbed Jun12682, inhibited several strains of the SARS-CoV-2 virus, including strains that resist treatment with Paxlovid.
Oral treatment with Jun12682 on SARS-CoV-2-infected mice was shown to reduce viral lung loads and lesions while improving survival rates.
“Our treatment was about as effective in mice as Paxlovid was in its initial animal tests,” said Wang, who added the experimental drug appears to have at least one major advantage over the older drug.
“Paxlovid interferes with many prescription medications, and most people who face the highest risk of severe COVID-19 take other prescription medicines, so it’s a real problem,” Wang said.
“We tested our candidate Jun12682 against major drug-metabolising enzymes and saw no evidence that it would interfere with other medications.”
Source: Rutgers University