Nearly 5000 Children are Living with Blood Cancer: The Toll on SA’s Caregivers
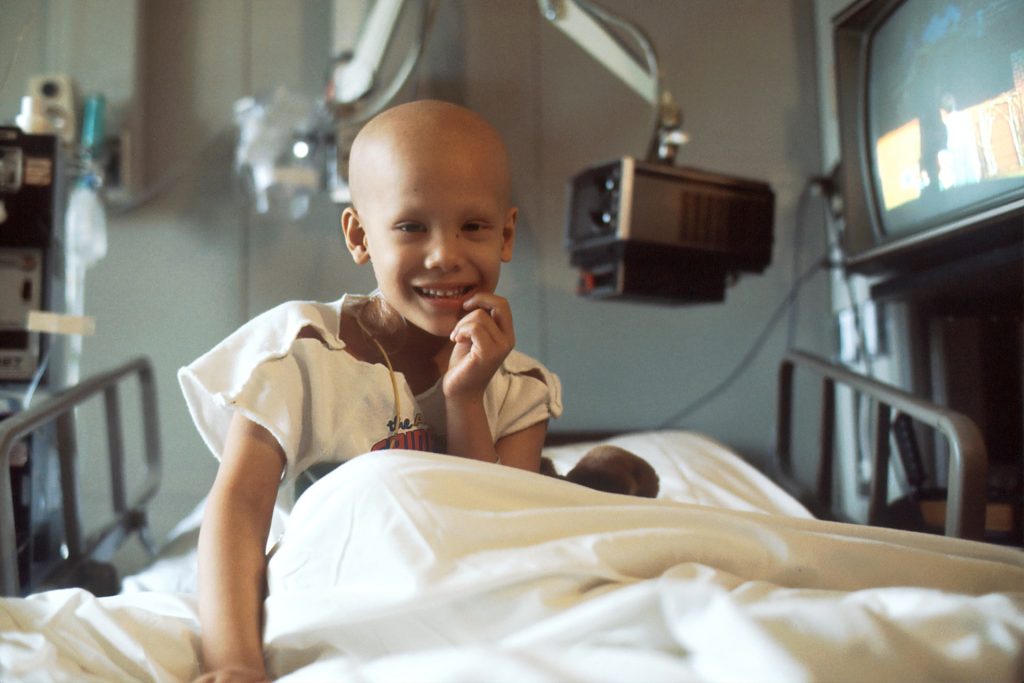
Blood cancer, a term covering several malignant diseases of the bone marrow or blood-forming system, accounts for 33% of all childhood cancers in South Africa. Currently, nearly 5000 children are living with the condition. For parents and caregivers, the emotional and financial strain can be overwhelming, often leaving them struggling to cope.
Ahead of International Childhood Cancer Day, on the 15th of February, Palesa Mokomele, Head of Community Engagement and Communications at DKMS Africa, explains that with the childhood cancer survival rate as low as 20%, a diagnosis is often a devastating blow for families.
“One thousand four hundred South African children are diagnosed with blood cancer annually,” she continues. “While the diagnosis is traumatic for the child, caregivers experience immense psychological distress which can severely impact their quality of life.”
A Mother’s Story
Elizabeth, whose son Ntsako was diagnosed with blood cancer in August 2024, describes the experience as “a bolt of lightning” that turned her world upside down. “I try not to cry in front of my son, even when I feel like I am falling apart. The treatment phase has been brutal. I want to stay strong for him but knowing there’s only so much I can do is heartbreaking.”
Mokomele notes that Elizabeth’s experience is shared by many. “Prolonged treatment, high stress, sleep deprivation, and financial strain take a heavy toll. Many caregivers struggle with anxiety, depression, and burnout, affecting their well-being, family dynamics, and social lives.”
Coping With the Emotional Impact
While every parent handles these challenges differently, there are ways to manage the emotional burden:
· Fear and anxiety: The unknown can be debilitating. Engaging with doctors and learning about the treatment and outcomes, which, while still stressful, can remove much of the uncertainty. Your child’s care team is not only there for your child but also to help you; enlist their support and lean on them.
· Denial and anger: In the short term, denial may help you adjust to the reality of your child’s diagnosis, but staying in denial for too long can cause isolation and delay treatment. Once this wears off, it can give way to anger, and without a proper outlet, it may build up inside. This can lead to you misdirecting it toward other loved ones, co-workers, and even doctors. Look for support from other parents who are going through the same process. Communicate your feelings with those close to you and explore ways to help you cope, like exercise, journaling, mindful meditation, or even just giving yourself private time to vent your feelings.
· Guilt and blame: It is natural to look for someone or something to blame. You may look inward to find something you think you did wrong; maybe you feel you didn’t act soon enough, or you’re angry that you didn’t get to the doctor earlier. Acknowledging these feelings and allowing yourself to process them is important. If these feelings become too overwhelming, seek support from a professional or even from your child’s care team.
· Sadness and loss: Give yourself the space to acknowledge grief and adapt to your new reality. If these feelings start to impact your ability to function, get support to work through them because they will affect your ability to help your child and other family members cope.
A Life-Saving Solution
More than 500 South African children die from blood cancer annually – a number that can be reduced with early detection and timely intervention.
“Blood cancer patients can often overcome the disease with the help of a stem cell transplant from a suitable donor,” highlights Mokomele. “DKMS provides a second chance at life for more than 22 patients every day, but doctors still struggle to find matches. Registering as a donor takes just five minutes but could save a child’s life and offer some much-needed relief for those caregivers who are doing their best to hold their families together.”
Register at https://www.dkms-africa.org/register-now.
For further information, get in touch with DKMS Africa at 0800 12 10 82.





