Scientists Find a Protein That Keeps Melanoma Hidden from the Immune System
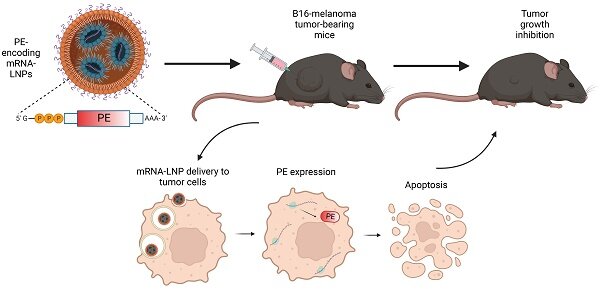
New research has helped explain how melanoma evades the immune system and may guide the discovery of future therapies for the disease. The study found that a protein known to be active in immune cells is also active inside melanoma cells, helping promote tumour growth. The findings, published in the journal Science Advances, suggest that targeting this protein with new drugs may deliver a powerful double hit to melanoma tumours.
“The immune system’s control of a tumour is influenced by both internal factors within tumour cells, as well as factors from the tumour’s surroundings,” says first author Hyungsoo Kim, PhD, a research assistant professor at Sanford Burnham Prebys in the lab of senior author Ze’ev Ronai, PhD. “We found that the protein we’re studying is involved in both, which makes it an ideal target for new cancer therapies.”
“Immunotherapy is the first-line therapy for several cancers now, but the success of immunotherapy is limited because many cancers either don’t respond to it or become resistant over time,” says Kim. “An important goal remains to improve the effectiveness of immunotherapy.”
To find ways to boost immunotherapy in melanoma, the research team analysed data from patient tumours to identify genes that may coincide with patients’ responsiveness to immunotherapy. This led to the identification of a protein that helps tumours evade the immune system – called NR2F6 – which was found not only in tumour cells, but also in the surrounding noncancerous cells.
“Often we find that a protein has the opposite effect outside of tumours compared to what it does within a tumour, which is less effective for therapy,” says Kim. “In the case of NR2F6, we found that it elicits the same change in the tumour and in its surrounding tissues, pointing to a synergistic effect. This means that treatments that block this protein’s activity could be twice as effective.”
In a mouse model, the researchers then deleted the NR2F6 protein in both melanoma tumours and in the tumours’ environment. This inhibited melanoma growth more strongly, compared to when this effect occurs in either the tumour or its microenvironment alone. The cancer’s response to immunotherapy was also enhanced upon loss of NR2F6 in both tumours and their microenvironment.
“This tells us that NR2F6 helps melanoma evade the immune system, and without it, the immune system can more readily suppress tumour growth,” adds Kim.
To help advance their discovery further, the team is working with the Institute’s Conrad Prebys Center for Chemical Genomics to identify new drugs that can target NR2F6.
“Discovering drugs that can target this protein are expected to offer a new way to treat melanomas, and possibly other tumours, that would otherwise resist immunotherapy,” says Kim.
Source: Sanford Burnham Prebys