Not Just for Respiration: Lungs Also Produce Blood Cells
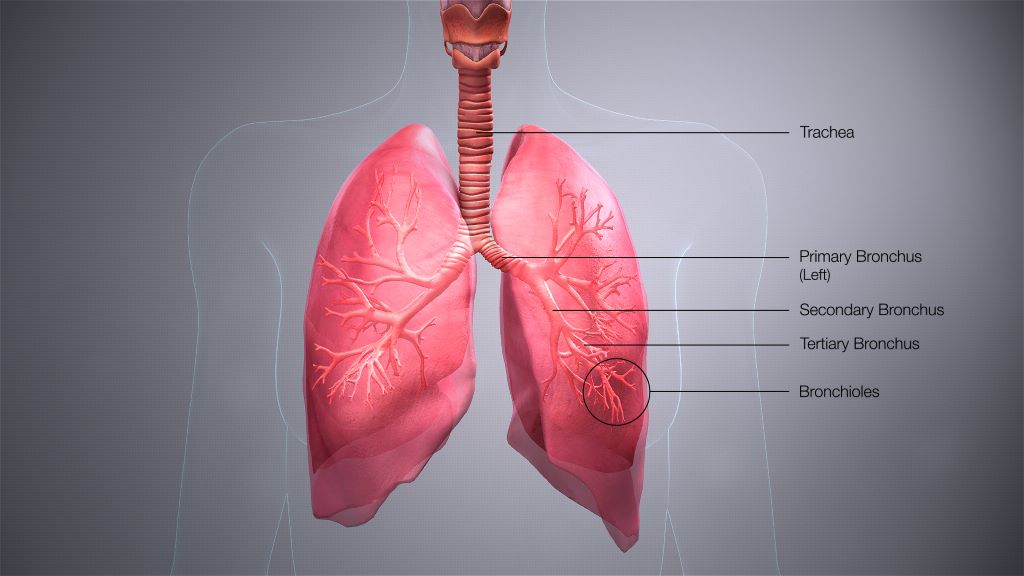
For many years, scientists assumed that blood production took place in the bone marrow, providing the 200 billion blood cells needed per day. But now, researchers at UCSF are showing it’s also happening in the lungs.
They found haematopoietic stem cells (HSCs) in human lung tissue that make red blood cells, as well as megakaryocytes, which produce the platelets that form blood clots. The findings appear in the journal Blood.
The work, which was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH), suggests the lungs could be a potent source for life-saving stem cell transplants.
“For decades, bone marrow transplants have been a lynchpin in the treatment of cancers like leukemia,” said Mark Looney, MD, professor of medicine and laboratory medicine at UCSF and senior author of the paper. “The lung HSCs could prove to be a second and significant reservoir of these precious stem cells.”
From mouse to human
In 2017, the UCSF team found cells in the mouse lung making 50% of the mouse’s platelets.
They also discovered lung stem cells in mice that made all the constituents of blood, including red blood cells, megakaryocytes and several types of immune cells.
Looney’s group wanted to prove this was also happening in people. So, they obtained donated samples of lung, bone marrow and blood, and compared what they found in each tissue.
Screening a golf-ball-sized volume of lung tissue, the scientists found stem cells in the lung that strongly resembled the well-known HSCs of bone marrow. Surprisingly, the HSCs were found at similar rates in both lung and bone marrow.
“The lung HSCs weren’t one-offs – they were a reliable presence in the lungs,” said Catharina Conrad, MD, PhD, postdoctoral scholar in Looney’s lab and first author of the paper. “But we still needed to know that they were actually capable of making blood.”
So, the scientists coaxed lung and bone marrow HSCs to mature in petri dishes and found the lung HSCs were productive just like the bone marrow HSCs.
“Both types of HSCs thrived in our gold-standard stem cell experiment, but the lung HSC colonies made more red blood cells and megakaryocytes, while the bone marrow colonies tended to make more immune cells,” Looney said.
The human lung HSCs also could restore bone marrow in HSC-deficient mice. The discovery confirmed Looney’s earlier discovery that the mouse lung and bone marrow complemented one another in producing blood, even sending stem cells to restore one another.
“We think these HSCs could be a reservoir of haematopoiesis in a particular organ, in this case the lung, that gets activated whenever the body needs more of any part of the blood, whether it’s platelets, red blood cells or immune cells,” Looney said.
Getting to know the new HSC in town
To show that the lung HSCs truly resided in the lung, and weren’t just escapees from the bone marrow, Conrad and Looney looked for the HSCs in human lung tissue samples.
They found them between blood vessels in an arrangement that was reminiscent of what’s seen in bone marrow.
“They really seem to live there and aren’t just passing through,” Conrad said.
Lastly, the team analysed the output of routine bone marrow transplants, which today begin with a blood draw from a donor followed by a screen for stem cells.
Remarkably, nearly a fifth of the stem cells isolated for bone marrow transplant carried the signature of lung HSCs – suggesting that cells in “bone marrow transplants” aren’t only from bone marrow.
There’s a lot more to learn about the lung HSCs. Could the different pools of HSCs serve different therapeutic roles in medicine? Why do the lungs themselves need to make blood?
“The lungs are critical to blood circulation, so it’s tantalising to see the lung HSCs as an emergency reservoir for red blood cell and platelet production,” Looney said. “Now that we know they exist, it opens up a lot of new opportunities for a therapy, hematopoietic stem cell transplantation, that is very commonly used for patients with the need.”
Source: EurekAlert!