Long-term Blood Pressure Control from Bariatric Surgery is Most Effective
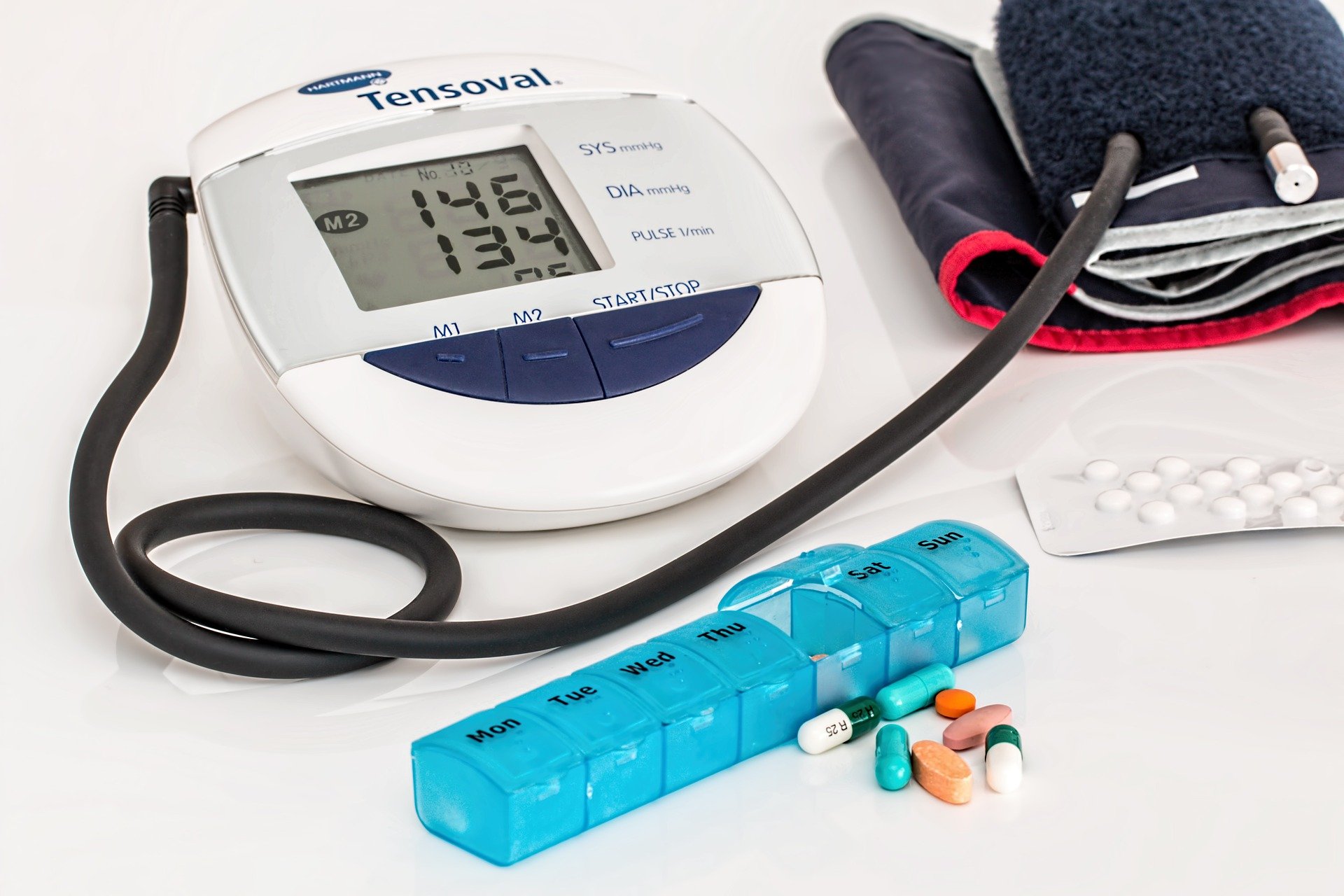
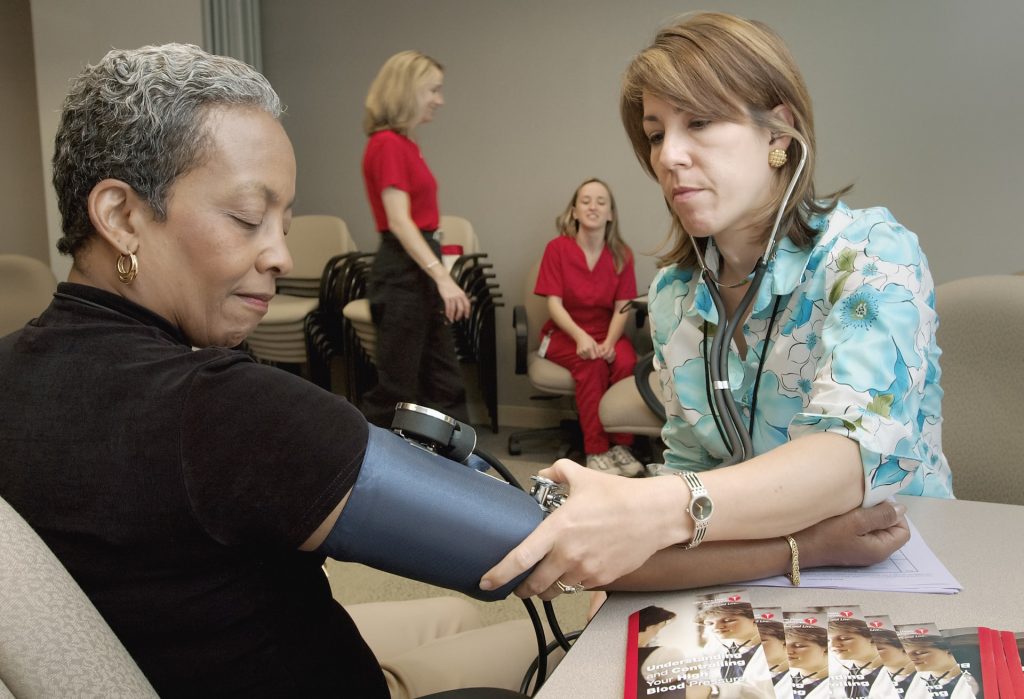
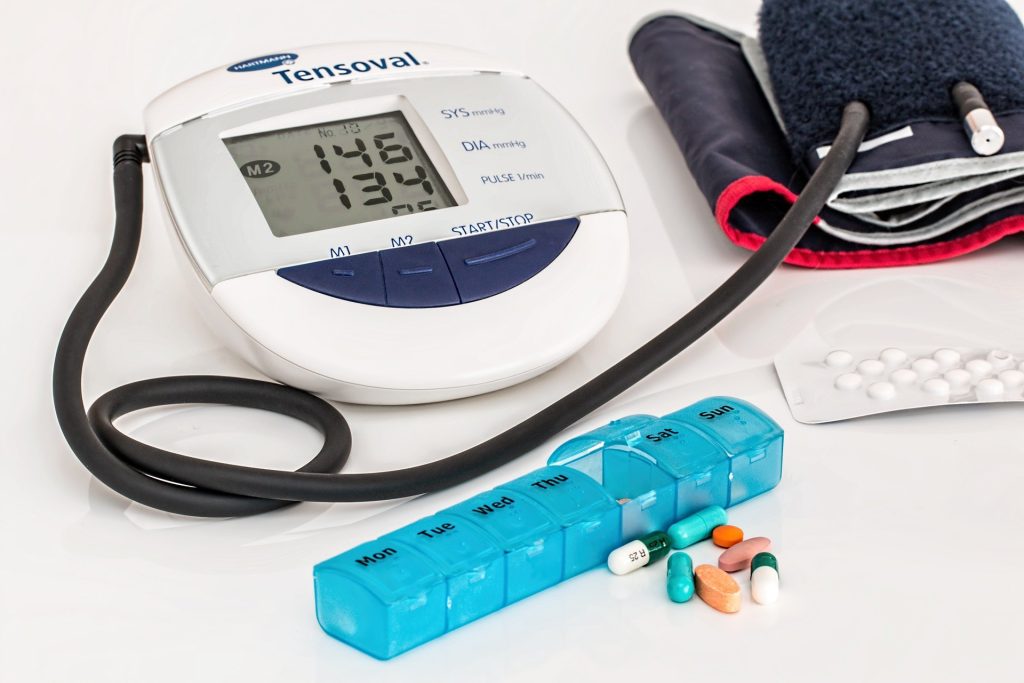
Compared to antihypertensives alone, bariatric surgery is more effective in controlling hypertension rates in people with obesity and uncontrolled hypertension, according to a study published in the Journal of the American College of Cardiology. People who underwent bariatric surgery had lower BMI and were on fewer medications after five years while maintaining normal blood pressure levels than those who only used antihypertensive medications.
“In clinical practice, obesity is an overlooked condition. As a consequence, there is a frequent failure in approaching obesity as a crucial step for mitigating the risk of important cardiovascular risk factors including hypertension,” said Carlos Aurelio Schiavon, MD, FACS, lead author of the study and a surgeon specialising in bariatric surgery at Heart Hospital (hcor) and BP Hospital in Sao Paulo.
Researchers in this study looked at the impact of treating obesity to lower hypertension. While new weight loss drugs exist, long-term adherence to medication can be challenging.
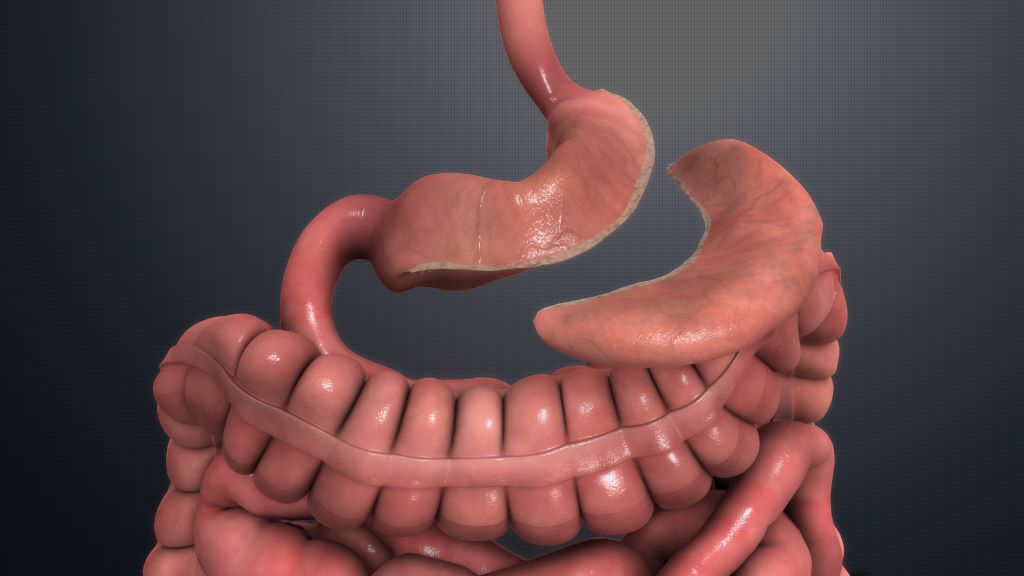
This study looks at bariatric surgery as a better long-term solution to control obesity and, as a result, hypertension.
The GATEWAY trial included 100 people (76% of whom were female) who had a body mass index (BMI) of around 36.9kg/m2. All participants had hypertension and were using at least two medications. People with previous cardiovascular events and poorly controlled Type 2 diabetes were excluded.
Subjects were assigned to either Roux-en-Y gastric bypass with medical therapy or medial therapy alone and the primary outcome was reduction of at least 30% antihypertensive medications while maintaining blood pressure levels less than 140/90mmHg at five years.
At five years, BMI was 28.01kg/m2 for those who received bariatric surgery and 36.40kg/m2 for those on medical therapy alone.
People who had bariatric surgery had an 80.7% reduction in the number of medications they were taking compared to a 13.7% reduction in those only using medical therapy.
Hypertension remission, defined as controlled blood pressure without medications, was 46.9% in those who underwent bariatric surgery compared to 2.4% in those on medical therapy alone.
“Our results underscore the importance of approaching obesity in reducing hypertension rates,” Schiavon said.
Limitations of the study include that it was a single-center, open-label study with a small sample size and there was loss of follow up in some patients.
In an accompanying editorial comment, Michael Hall, MD, MSc, professor and chair of the Department of Medicine at the University of Mississippi Medical Center, said the study provides important long-term data on the benefits of gastric bypass on weight loss and blood pressure control, but questions remain.
“Further studies assessing the threshold for bariatric surgery in people with obesity, optimal timing of bariatric surgery in obese people with cardiometabolic diseases, type of bariatric surgery and comparative studies of obesity pharmacotherapies and bariatric surgery are needed to clarify the optimal treatment pathways for this common and growing disease,” he said.
Source: American College of Cardiology