Healthy Cells Can Migrate Just like Metastatic Cancer Cells
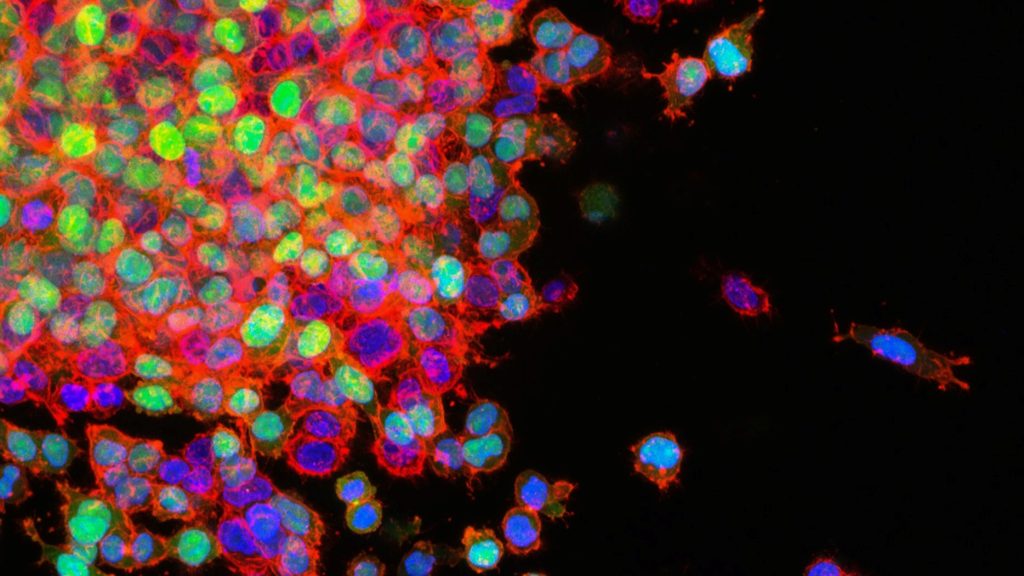
Cambridge University scientists have discovered that cancer cells ‘hijack’ a process used by healthy cells to spread around the body, challenging how cancer metastasis is currently understood. Publishing in Nature Genetics, the team found that blocking the activity of a sodium channel protein in cells in mice with cancer triggers metastasis.
To their surprise, the investigators also discovered that this process extends beyond just cancer: when they removed NALCN from cancer-free mice, this caused their healthy cells to leave their original tissue and travel around the body where they joined other organs.
They found, for example, that healthy pancreas cells migrated to the kidney where they became healthy kidney cells. This suggests that metastasis isn’t an abnormal process limited to cancer as previously thought, but is in fact a normal process used by healthy cells that has been exploited by cancers to generate metastases.
Group Leader of the study, Professor Richard Gilbertson, said: “These findings are among the most important to have come out of my lab for three decades. Not only have we identified one of the elusive drivers of metastasis, but we have also turned a commonly held understanding of this on its head, showing how cancer hijacks processes in healthy cells for its own gains. If validated through further research, this could have far-reaching implications for how we prevent cancer from spreading and allow us to manipulate this process to repair damaged organs.”
Metastasis has been challenging to treat because of the difficulty in identifying key drivers of this process that could be targeted by drugs. Now that they have identified NALCN’s role in metastasis, the team are looking into various ways to restore its function, including using existing drugs on the market.
Lead researcher on the study Dr Eric Rahrmann, said: “We are incredibly excited to have identified a single protein that regulates not only how cancer spreads through the body, independent of tumour growth, but also normal tissue cell shedding and repair. We are developing a clearer picture on the processes that govern how cancer cells spread. We can now consider whether there are likely existing drugs which could be repurposed to prevent this mechanism from triggering cancer spreading in patients.”
NALCN stands for sodium (Na+) leak channel, non-selective. Sodium leak channels are expressed predominately in the central nervous system but are also found throughout the rest of the body, controlling the amount of sodium that goes in and out of the cell. Controlling this process also alters the balance of electricity across the cell membrane. It is still unclear why these channels seem to be implicated so directly in cancer metastasis.
Source: University of Cambridge