Can People Accurately Assess the Strength of Their Immune Systems?

People often say whether they feel like their immune system is ‘down’ – but could there be some truth to this? A recent study showed that when freshly vaccinated people self-assessed the strength of their immune response, their estimates correlated well to their measured antibody levels. They were even more accurate when their immune response was weak. The results were published in the journal Biological Psychology.
At the University of Konstanz, Stephanie psychologist Dimitroff researches the connection between our brain and our immune system. “Listen to your body,” she concludes from her study. “The field of medicine is moving towards greater patient orientation. Our findings support the idea that patients’ self-perceptions provide valuable clues about their state of health. Physicians should listen to them more.”
Communication between the immune and nervous systems
One part of our brain, the insula, receives information from the body and gives us a basic impression of its condition, which until now was assumed to be quite general in nature. Stephanie Dimitroff’s study now suggests that our brain can perceive the body’s condition more specifically than previously thought. Is it possible that our brain can assess the state of our immune system?
“Of course, our brain does not count antibodies. But our immune system is intrinsically connected to the central nervous system,” Dimitroff explains. “The immune system is regulated via this connection. And our brain also receives information from the immune system.”
This communication between the immune system and the central nervous system is key for our sense of well-being or illness. “It is important to know here: When we feel ill, for example, we have a cold, this feeling is caused quite significantly by the immune system’s communication with the central nervous system,” says Dimitroff. “The brain receives signals that something is wrong with the body and causes the feeling of illness as a result.”
The same flow of information between the immune and nervous systems can generally also take place when the body is not ill. This means it could be possible that this communication process gives us an impression of our immune system even when we are healthy. Stephanie Dimitroff’s study investigates whether this is actually the case.
Results of the study
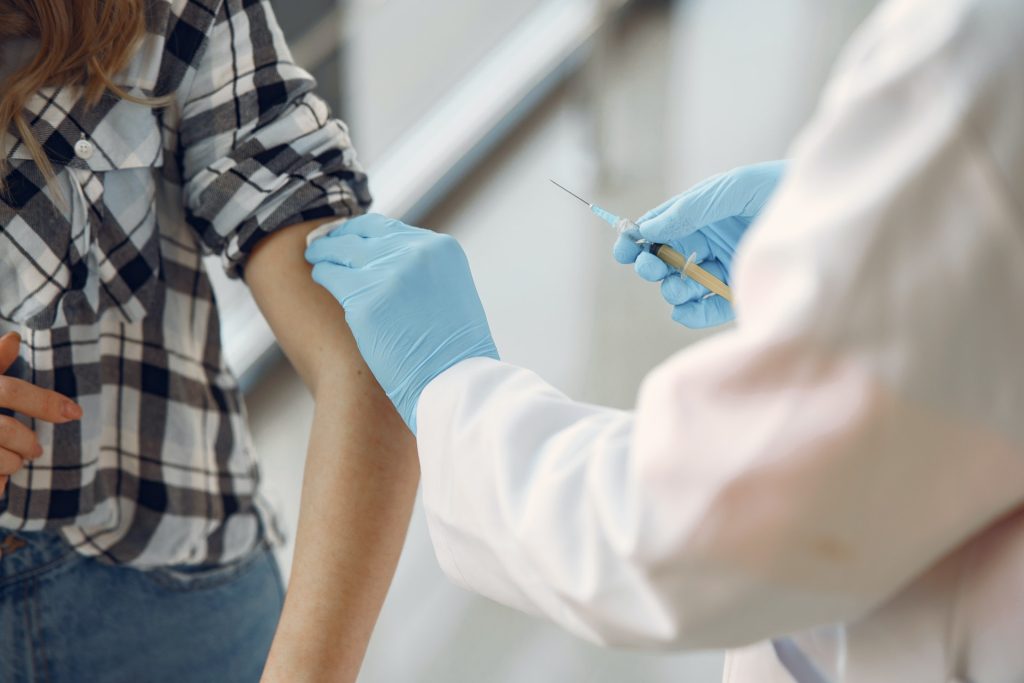
The study looked at people who had received the COVID-19 vaccine. This group of participants was chosen because a particularly large number of people received the vaccine in the summer of 2021, when the study was conducted. 166 people between the ages of 18 and 59 participated in the study.
After vaccination, the participants in the study were able to assess surprisingly well how strongly their immune system was positioned to fight the respective illness. This was especially true for people who had developed only a few antibodies. In fact, 71% of participants who did not feel well protected after vaccination also had a below-average immune response. “Our most notable finding is that those who felt they had not produced high levels of antibodies after vaccination were often correct in their assessment.”
By contrast, participants who assessed their immune response as good were not always right. However, all of those who had a particularly strong immune response also reported feeling well protected.
Alternative interpretations
For Stephanie Dimitroff, however, it is still too early to draw any final conclusions. The psychologist is considering other possible causes, including the placebo effect. This is because communication between the brain and the immune system runs in both directions. The signals from our brain can therefore also influence our immune system. People who firmly believe in vaccination or are basically optimistic could thus actually develop a better immune defence (placebo effect) and also feel better protected. It is therefore possible that belief in the effectiveness of a vaccine is what improves its efficacy, and this could also explain the high accuracy of the self-assessments.
“Our results suggest that it is quite likely that people have a real ability to assess their own health. However, I cannot rule out that there is a combination of effects at play, including the placebo effect and/or feelings of optimism,” Dimitroff says. In her view, it would make sense to repeat the study in order to confirm the results and rule out alternative causes.
Source: University of Konstanz