Christmas Came Early with These FDA Approvals

After the stupendous effort for COVID vaccines and treatments, it may seem like other diseases were being neglected. Nevertheless, the US Food and Drug Administration suddenly had a fire lit underneath it, and got cracking with accelerated drug approvals. Now, 2023 seems to have brought plenty of new drugs to bolster the physician’s armamentarium – some are the first-ever treatment for their indications. Hopefully, with FDA and European Medicines Agency (EMA) approvals, South African approvals should not be too far behind.
Since the pandemic, hotly anticipated drugs have made a big splash or sunk without a trace. In 2021, semaglutide was approved for weight management, unleashing a wave of people using (and some abusing) the GLP-1 agonist for weight loss. Adagrasib, which targets KRAS, previously thought undruggable, was a major advance for the treatment of non-small-cell lung cancer and was one of a few notable new non-COVID pharmaceuticals.
Aducanumab/Aduhelm was the top tip for new drugs in 2021, but turned out to be an absolute debacle: it wound up being an astronomically expensive, mostly ineffective drug with significant side effects. There were even questions raised over how it got approved in the first place.
Alzheimer’s disease
Last year, Aduhelm seemed like yet another false start in the long battle against Alzheimer’s disease. This year though, it looks like help finally arrived for fight against the dreaded neurodegenerative disease with not one but two breakthrough drugs, both antiamyloid antibodies.
Up first is lecanemab/Leqembi from Eisai/Biogen. It targets the buildup of amyloid proteins in the brain, which otherwise lead to the formation of amyloid plaques and neurofibrillary tangles of tau protein, the hallmarks of the disease.
The other candidate is donanemab, which did not secure FDA approval last year, after pharma company Eli Lilly witnessed the disaster that was Aduhelm. It did show a reduction in decline in one measure of Alzheimer’s disease but not another, so its effects are a mixed bag.
Like Aduhelm, donanemab and lecanemab both have a serious downside: brain swelling, which claimed the lives of at three donanemab trial participants.
RSV
Previously minimised by the pandemic’s social distancing and routine masking, respiratory syncytial virus (RSV) experienced a resurgence in the wake of lifting these restrictions. RSV afflicts primarily those over 60 and young children. Among those 65 and older with RSV in the US, the Centers for Disease Control estimated 120 000 annual hospitalisations, with up to 10 000 of whom dying. Among children under 5, the figures are 58 000 annual hospitalisations and 100 to 300 deaths. Historically, RSV vaccine developments wound up being ineffective. Fortunately, this year saw the first approval for an RSV vaccine. A 120µg dose of their Arexvy vaccine produced statistically significant and clinically meaningful reductions in cases of lower respiratory tract disease caused by RSV in adults aged 60 years and older. Pfizer and Moderna are hot on HSK’s heels with their own vaccine applications.
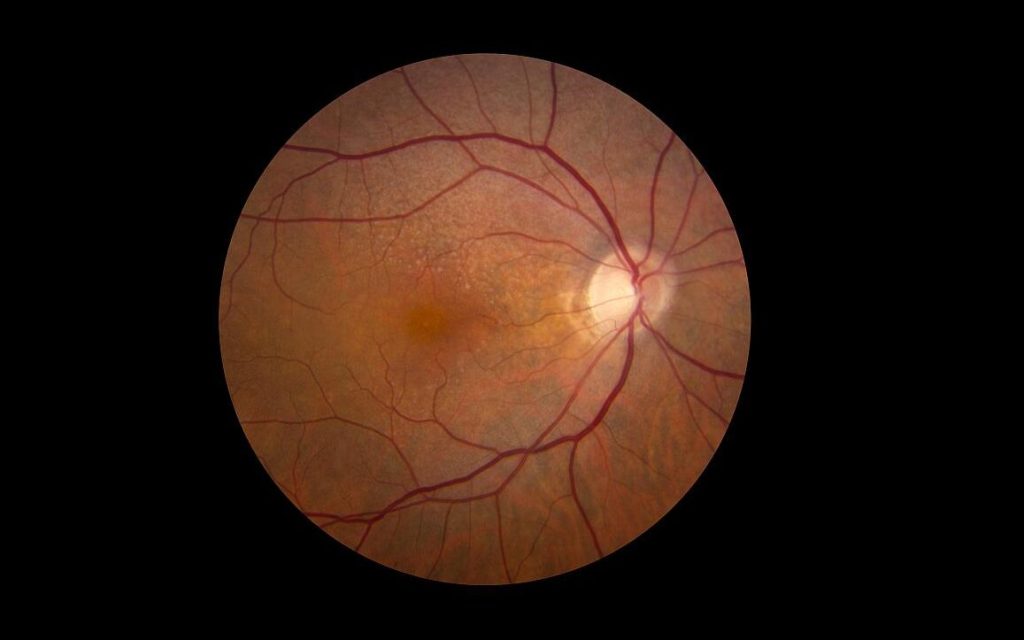
Age-related macular degeneration
Apellis got an approval for pegcetacoplan this year, for geographic atrophy (GA) secondary to age-related macular degeneration, in its intravitreal injection. This is the first and so far only treatment for this indication. “The approval of SYFOVRE is the most important event in retinal ophthalmology in more than a decade,” said Eleonora Lad, MD, PhD, lead investigator for the phase 3 study. “Until now, there have been no approved therapies to offer people living with GA as their vision relentlessly declined. With SYFOVRE, we finally have a safe and effective GA treatment for this devastating disease, with increasing effects over time.”
Interestingly, Apellis also got an approval for paroxysmal nocturnal haemoglobinuria (PNH) with a patient-injectable version of pegcetacoplan. The disease results from the destruction of red blood cells by the immune system.
Lymphoma
Abbvie and Genmab’s epcoritamab, for certain cases of large B-cell lymphoma (LBCL), got accelerated FDA and EMA approval earlier this year. The FDA has also granted accelerated approval to Roche’s glofitamab. The drugs bind to binding to CD20 on malignant B cells and CD3 on T cells to kill cancer cells, creating an effect like CAR-T cell therapy but without the complexity (and presumably, cheaper too).
Major depressive disorder, postpartum depression
Mental health is full of gaps needing to be filled by effective treatments. Not much has made been added for depression since selective serotonin reuptake inhibitors (SSRIs) came onto the market in the 1990s. Zuranolone, from Biogen and Sage Therapeutics, is the first oral treatment for postpartum depression, which previously was treated only by IV injection in a healthcare facility. Unlike slow-acting SSRIs, this treatment, which targets the GABA-A receptor, is a short course.
Inflammatory bowel disease
There has been a steady drip of new biologic drugs for inflammatory diseases, such as bimekizumab (psoriasis and deucravacitinib which recently received FDA approval. Eli Lilly entered this crowded marketplace with ixekizumab. Now, after trouncing Novartis’ Cosentyx for psoriasis with its own mirikizumab, it pulled its application for that indication and switched it to ulcerative colitis – beating about a dozen competitors to be the first IL-23 inhibitor. It aims to get an approval for Crohn’s disease in 2025. Pfizer’s etrasimod for ulcerative colitis got approval in October 2023, and should receive EMA approval in 2024. Its phase 3 trial achieved 27% remission versus 7.4% for placebo.
Pulmonary arterial hypertension
Last is sotatercept, a new drug for pulmonary arterial hypertension (PAH), which previously had no real treatment. Unlike the current therapy aimed at simply dilating blood vessels, sotaracept targets BMPR-II signalling, addressing the cause of PAH. It earned a priority preview by the FDA based on its phase 3 trial data, with possible approval by March 2024.