Why the Road for New Heart Cell Treatments is so Long
Pathways to new treatments for heart failure take time – as long as four decades for two now accepted therapies. So, new attempts to repair scar tissue in infarcted hearts using cells or cell products need more time to develop clinical therapies that can reduce risk of death from heart failure after a heart attack.
This message is part of a critical review of cell-based and cell product-based therapies for the treatment of heart failure. The review details 20 years of completed and ongoing clinical trials. While none has gained medical approval, they have proven safe and some have shown beneficial effects.
More importantly, the reviewers note, it took longer, nearly 40 years, to optimise two current therapies to reduce mortality in heart failure: implantable cardioverter–defibrillators and guideline-directed medical therapy.
“The history of the development of life-saving medical therapies for heart failure serves as an important lesson that we should remain hopeful of the promise of cell therapy in heart failure,” Jianyi “Jay” Zhang, MD, PhD, and colleagues write in the review, “Trials and tribulations of cell therapy for heart failure: an update on ongoing trials,” published in Nature Reviews Cardiology. Zhang is professor and chair of the University of Alabama at Birmingham Department of Biomedical Engineering.
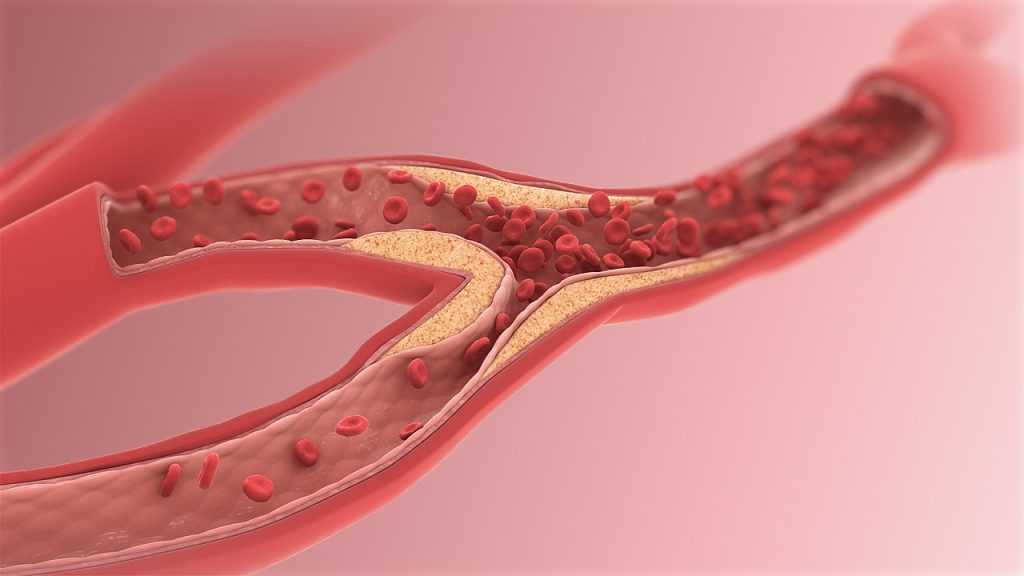
Heart failure is responsible for 13% of deaths worldwide. Half of patients with heart failure die within five years. The most common cause of heart failure is blockage of coronary arteries leading to death of the cardiomyocyte heart muscle cells. When that muscle tissue is replaced by dense scar tissue with little blood circulation, the infarcted heart loses contractile power, leading to heart enlargement, progressive loss of pumping ability, increased chance of ventricular arrhythmias and clinical end-stage heart failure.
The problem is that shortly after birth, human heart muscle cells lose their ability to divide, so a damaged infarcted heart cannot repair itself by growing new muscle cells. Thus, the simple idea behind initial cell therapies was to add or inject replacement cells to the scar area to restore muscle tissue.
The two decades since has been a long road, with bumps and turns. The three parts of the Nature Reviews Cardiology paper describe the journey.
First is a history of the slow development, obstacles, setbacks and scepticism for two current heart failure therapies, implantable cardioverter–defibrillators and guideline-directed medical therapy. The next two sections, and main focus of the review, survey 13 completed clinical trials published in the last 12 years and 10 very recently initiated and ongoing clinical trials that are based on the lessons learned from the past 20 years of research, to assess the safety and efficacy of cell- and cell products-based therapy approaches.
While several randomised, double-blind, multicentre phase II or III trials published in the past 20 years support the concept that even a single dose of cell products has beneficial effects in patients with heart failure on optimal medical therapy, the ongoing trial are taking novel directions, Zhang says.
These include:
- New cell types — pluripotent stem cell-derived cardiomyocytes/ spheroids and umbilical cord-derived mesenchymal stem cells
- Repeated intravenous injections as a noninvasive cell delivery method
- New cell products, such as engineered epicardial cardiomyocyte patches
- Novel cell-free products — extracellular vesicle-enriched or exosome-enriched secretomes.
“The results of these trials will continue to define and refine our understanding of cell and cell product therapy as a novel addition in the treatment of patients with heart failure,” Zhang said.
The review acknowledges scientific criticism during the slow but consistent progress and evolution of cell therapy. Some have questioned the use of public funding to support cell therapy research for heart failure treatment, due to poorly designed or underpowered clinical trials and very modest improvements in cardiac function in preclinical studies that are not always substantiated in large-scale clinical trials.
“These criticisms must be addressed in future trials that are adequately powered and rigorously designed to ensure continued progress of the field,” Zhang said. “Critique is an essential part of science, and the basis for growth, innovation and evolution – this is no less true for the field of cell therapy.”
Yet Zhang is confident that current research will yield clinical translation. “In the past 20 years, cell therapy has emerged and evolved as a promising avenue for cardiac repair and regeneration,” he said. “Cell therapy has encountered substantial barriers in both preclinical studies and clinical trials, but the field continues to progress and evolve through lessons learned from such research.”