Radiology’s Role in the Diagnosis and Management of Diabetic Complications

Radiology provides crucial insights into the complications caused by diabetes, allowing for timely diagnosis, effective management and monitoring of disease progression. Early detection of these complications can significantly improve patient outcome and quality of life.
What is diabetes?
Diabetes is known as a ‘silent killer’ because it is quite often asymptomatic at the onset. Diabetes, a major lifestyle disorder, has become one of the most dangerous and common diseases in the world. It is a chronic disease that causes high blood sugar levels and occurs when the body doesn’t produce enough insulin or use insulin properly.
Types of diabetes
- Type 1 diabetes: The body’s immune system destroys the cells that produce insulin
- Type 2 diabetes: The body doesn’t produce enough insulin or the body’s cells don’t react to insulin as they should
- Gestational diabetes: Sometimes occurs during pregnancy when the placenta releases hormones that cause insulin resistance. This tampers with the expectant mom’s blood sugar level, changing the amount of glucose in the blood
Around 4.2 million people in South Africa have diabetes – 90% of whom have type 2 diabetes, a lifestyle disease exacerbated by dietary factors, coupled with too little physical activity and high levels of obesity.
Dr Jean de Villiers, senior partner and radiologist at SCP Radiology, discusses the imaging techniques used to identify and manage complications of diabetes.
Cardiovascular Disease: People with diabetes are at higher risk of developing heart disease and other cardiovascular problems. Imaging techniques such as CT angiography can be used to assess the heart’s blood vessels and detect issues such as atherosclerosis, coronary artery narrowing or blockage of the arteries. CT angiography is also used for the neck, arm and leg arteries, as well as the arteries to the gut.
Stroke: Diabetes increases the risk of stroke by damaging blood vessels through high blood sugar levels, leading to the formation of fatty deposits and clots within the arteries. This can increase the chance of clot formation and block blood flow to the brain and cause a stroke. Imaging techniques such as MRI, CT scans, and ultrasound may be able to detect these fatty deposits in the arteries. The deposits are generally seen as areas of narrowing in the involved arteries or calcification of the walls of the arteries.
Blood vessel damage: Chronic high blood sugar levels can directly damage the lining of blood vessels, making them more susceptible to inflammation and clot formation. Essentially, the excess glucose in the blood weakens and stiffens the blood vessel walls, making them more prone to blockages. CT or MRI scans can be critical in identifying and assessing strokes, transient ischemic attacks (TIAs) or other cerebrovascular issues in diabetic patients.
High blood pressure association: People with diabetes often also have high blood pressure, which can exacerbate the damage to blood vessels and increases stroke risk. A CT of the coronary arteries is used to visualise blockages in the coronary blood vessels and assess the severity of atherosclerosis in diabetic patients. This helps in planning for interventions like stent placement or bypass surgery.
Kidney disease: Diabetes affects your kidneys by potentially damaging the blood vessels within the kidneys due to high blood sugar levels. This can lead to impaired kidney function, causing the kidneys to leak protein into the urine and eventually progressing to chronic kidney disease if left uncontrolled. This condition is often referred to as ‘diabetic nephropathy.’
Diabetic nephropathy can lead to kidney damage and radiology plays a role in assessing kidney size, structure and function. Renal ultrasound can help assess kidney size and detect signs of chronic kidney disease (CKD). In advanced cases, a CT scan or MRI can be used to further evaluate the kidneys for the presence of complications such as renal artery stenosis or renal scarring.
Diabetic neuropathy: Diabetic neuropathy is a complication of diabetes where high blood sugar levels damage nerves throughout the body. Most commonly affected are the nerves in the legs and feet, leading to symptoms like numbness, tingling, pain and sometimes muscle weakness. It can also impact internal organs, depending on which nerves are affected and is considered a serious diabetes complication that can affect up to 50% of diabetics.
While radiology is not typically used for direct diagnosis of diabetic neuropathy, it can help rule out other causes of neuropathy. MRI and CT scans can assess for structural issues, such as spinal problems or other nerve impingements that may be contributing to symptoms.
Infections: Diabetic patients have a higher susceptibility to infections due to impaired immune response.
Diabetic foot ulcers and infections: Over time, high blood sugar levels damage nerves, blood vessels and skin in the feet. Damaged nerves can cause loss of feeling in the feet, while damaged blood vessels slow blood flow to the feet, preventing the healing of injuries.
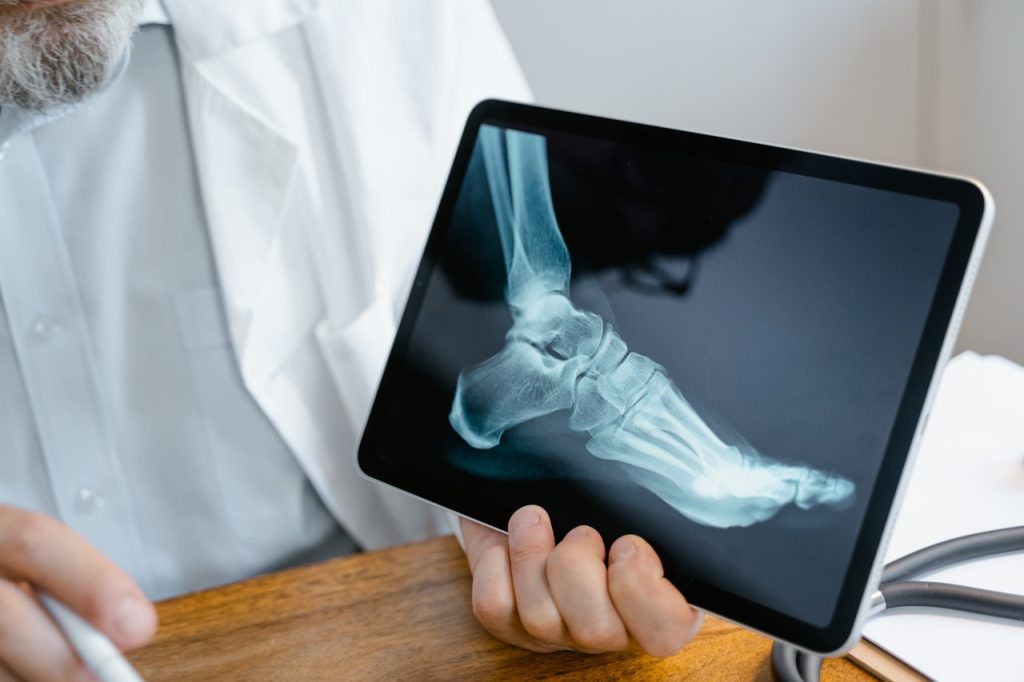
Imaging techniques like CT, MRI and ultrasound are useful for detecting and monitoring bone and soft tissue infections. These can be critical for determining the appropriate course of antibiotic treatment or surgical intervention. X-rays, CT and MRI can be used to assess for infection in diabetic foot, such as ulcers, osteomyelitis or abscesses that may progress to amputation if left untreated.
Liver disease: Non-alcoholic fatty liver disease (NAFLD) is commonly seen in diabetic patients. Ultrasound is the primary tool for detecting fatty liver, while CT and MRI may offer further details on liver fat content or cirrhosis. Regular monitoring through imaging can help prevent more severe liver damage.
Osteoporosis: Long-term diabetes, especially type 1, can increase the risk of osteoporosis due to lower bone density. A DEXA scan helps assess bone mineral density (BMD), aiding in the early detection of osteoporosis and providing information on fracture risk.
‘As with any lifestyle disease, prevention is best. However, second to this is early detection and timely diagnosis, effective management and monitoring of the disease,’ says Dr de Villiers. ‘In the case of diabetes, we work with physicians and patients to detect possible complications early enough to help improve medical care, monitor treatment response and ultimately, improve quality of life.’