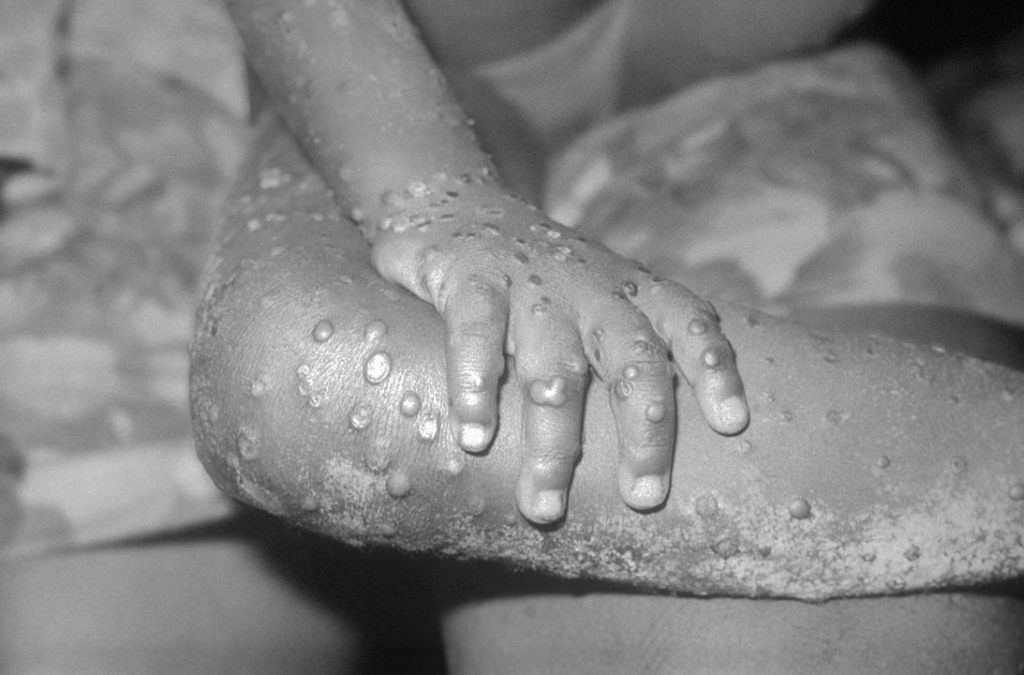
Asymptomatic Detection of Monkeypox Suggests it is More Widespread
A brief research report in Annals of Internal Medicine documents positive monkeypox virus PCR results found in anal samples taken from asymptomatic MSM (men who have sex with men). These findings suggest that vaccination limited to those with known exposure to the monkeypox virus may not be an effective strategy for preventing infection.
The findings come as the World Health Organization has renamed the variants, or clades, of monkeypox from their previous geographically-derived names to Roman numerals, eg, the former Congo Basin (Central African) clade is now Clade one (I). It is also seeking inputs on a possible new name for the virus in order to avoid stigmatisation.
Researchers from Bichat–Claude Bernard Hospital, Paris, retrospectively performed testing for monkeypox virus on all anorectal swabs that were collected as part of a sexually transmitted infection screening program. This type of screening is performed every three months among MSM with multiple sexual partners who are either taking HIV preexposure prophylaxis (PrEP) or living with HIV and receiving antiretroviral treatment. Of the 200 asymptomatic persons screened that were negative for N. gonorrhoeae and C. trachomatis, 13 (6.5%) samples were PCR positive for monkeypox virus. Two of the 13 later developed symptoms of monkeypox.
While it is not know whether asymptomatic transmission will play a role in the current worldwide monkeypox epidemic and the mode of human-to-human transmission may provide evidence that asymptomatic or preclinical spread can occur. In an accompanying editorial, Stuart N. Isaacs, MD, at the University of Pennsylvania, suggests that an expanded ring vaccination strategy and other public health interventions in the highest-risk communities are likely needed to help control the outbreak.
Source: EurekAlert!