CVD and Obesity: When Protective Lipids Decline, Health Risks Increase
New research from Weill Cornell Medicine has uncovered a surprising culprit underlying cardiovascular diseases in obesity and diabetes—not the presence of certain fats, but their suppression. The study, published in Nature Communications, challenges the conventional belief that a type of fat called ceramides accumulates in blood vessels causing inflammation and health risks. Instead, their findings reveal that when ceramides decrease in endothelial cells lining blood vessels, it can be damaging and cause chronic illnesses. Ironically, the findings could ultimately lead to therapies that maintain high levels of these protective lipids in patients with obesity.
Ceramides are found throughout the body and in the endothelium, the thin lining inside blood vessels. These waxy lipids regulate blood vessel tone, dilating or contracting vessels to modulate blood pressure. They also help prevent blood clots, keeping blood flowing easily through the body’s extensive highway of arteries and veins.
“The common assumption in the field was that high levels of ceramides in the endothelium of blood vessels contributed to cardiovascular disease, but this conclusion was extrapolated from in vitro data in cells,” said Dr Annarita Di Lorenzo, professor of pathology and laboratory medicine at Weill Cornell Medicine. “Ours is the first in vivo study that measures the levels of the lipids in the endothelial cells of an animal model. In obese mice fed a high-fat diet, ceramides do not build up—they decrease compared to lean mice.” Also working on this research are co-first authors Dr Onorina L. Manzo, postdoctoral associate and Luisa Rubinelli, both in Dr Di Lorenzo’s lab.
Ceramide to the Rescue
Dr Di Lorenzo and her team discovered the importance of ceramides in blood vessels two years ago. Together with Dr Giuseppe Faraco, assistant professor of neuroscience at Weill Cornell Medicine, they found that decreased levels of ceramides in otherwise healthy mice causes severe blood vessel inflammation in the brain, clot formation and death. Last year, the team reported that ceramide production increases as a protective response in a mouse model of coronary artery disease. Ultimately, when ceramide is broken down by the body it produces a compound called sphingosine-1-phosphate (S1P), which builds up and protects mice against cardiovascular disease. But when this process doesn’t work the mice are left vulnerable.
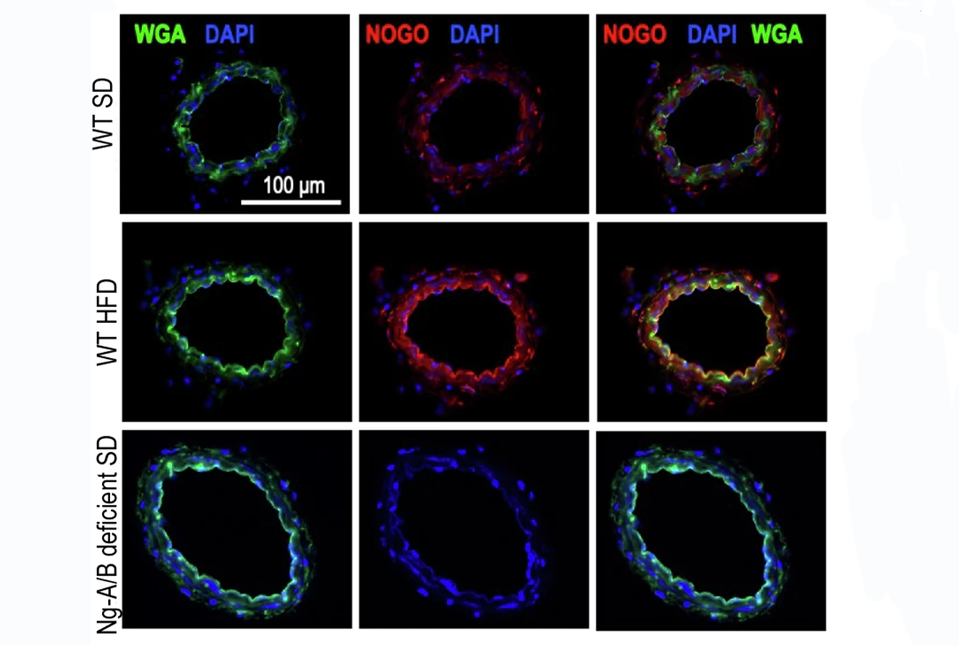
The researchers also found that two proteins, Nogo-B and ORMDL, decreased the production of ceramides and S1P in obesity. This decrease leads to increased blood pressure, impaired vascular regulation and higher glucose levels—all of which contribute to cardiometabolic conditions that affect the heart (cardiovascular system) and energy processing (metabolism), like diabetes like diabetes, hypertension, coronary artery disease and stroke.
Maintaining Balance
To understand how these different molecules interact, the researchers tested what happens in animal models. Mice with obesity fed a high-fat diet had low levels of ceramides and S1P, but high levels of Nogo-B. These mice showed signs of inflammation, diabetes and high blood pressure.
But what happens if the Nogo-B inhibitor wasn’t present? The researchers knocked out Nogo-B only in the endothelium of blood vessels in a mouse model to find out. “These mice have the same body weight and diabetes as controls, but their blood vessel health is much better,” said Dr. Di Lorenzo. “By knocking out this inhibitor, we preserved vascular health. This also showed that the regulation of ceramide metabolism causes vascular dysfunction and inflammation in obesity.”
The paper suggests that targeting this metabolic pathway could have multiple beneficial effects in the treatment of cardiometabolic diseases related to obesity. “Nogo suppresses biosynthesis of ceramides, so if we can identify a drug that can block Nogo-B, we could restore ceramide levels to a healthy balance and this would fight not only obesity and diabetes, but would directly keep blood vessels functioning properly,” she said.
Source: Weill Cornell Medicine