Transperineal Prostate Biopsy is Safer than the Standard Technique
A multi-institutional clinical trial led by Weill Cornell Medicine and NewYork-Presbyterian investigators showed that a newer technique for collecting prostate biopsy samples reduced the risk of infection compared with traditional biopsy approaches and removed the need for prophylactic antibiotics. The results of the study appear in JAMA Oncology.
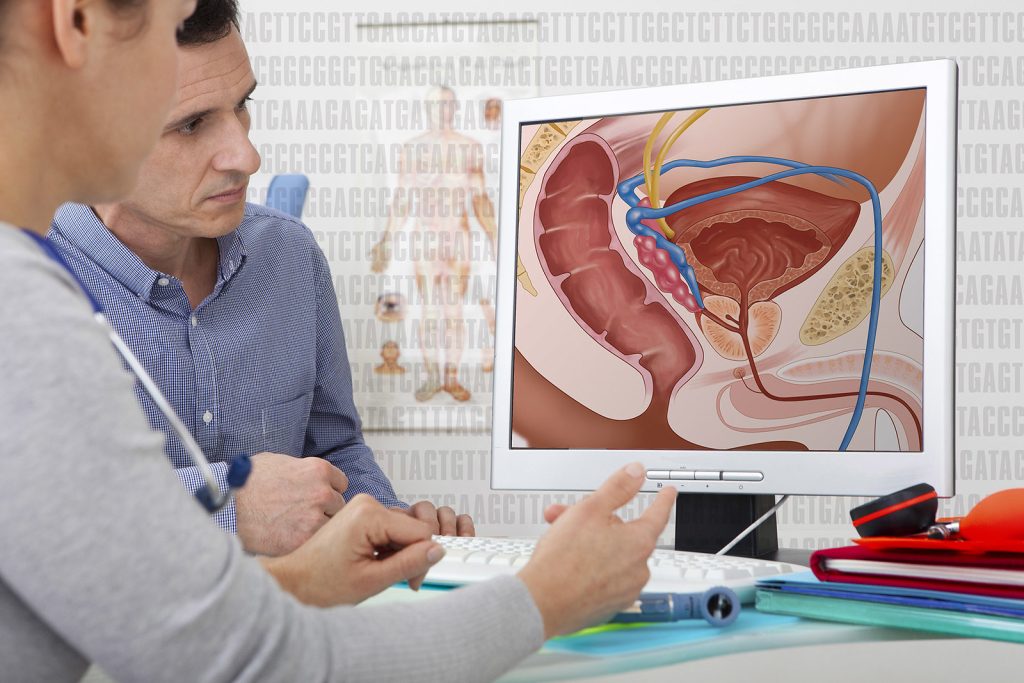
The technique, called transperineal prostate biopsy, collects prostate tissue via a needle through the skin of the perineum, the area between the rectum and the scrotum. The procedure, which uses local anesthesia to numb the area, allows physicians to bypass the traditional and more infection-prone route of collecting prostate biopsy tissue with a needle through the rectum.
The PReclude infection EVEnts with No prophylaxis Transperineal (PREVENT) trial, funded by the National Cancer Institute, part of the National Institutes of Health, was conducted at multiple sites, including NewYork-Presbyterian/Weill Cornell Medical Center, NewYork-Presbyterian Queens and NewYork-Presbyterian Brooklyn Methodist Hospital. The study found no infections among 382 men randomised to undergo the transperineal procedure compared with six infections affecting 1.6% of the 370 men randomised to undergo the traditional transrectal biopsy procedure. The lower infection rate is particularly remarkable because the men in the transrectal biopsy group received a targeted course of antibiotics designed to help reduce their infection risk, and the men in the transperineal group received no antibiotics.
“Transperineal biopsy should be the new standard of care for prostate biopsy,” said Dr Jim Hu, Professor of Urologic Oncology at Weill Cornell Medicine. “It was as effective as the traditional transrectal biopsy approach at detecting cancer, but without the risk of infection or the need for antibiotics.”
Prostate biopsies are an essential tool for detecting prostate cancer, and about 3 million people worldwide undergo the procedure each year. Dr Hu noted that physicians collect about 90% of these biopsies in the United States via a transrectal procedure. Yet studies have found that 5% to 7% of patients develop infections after biopsy, and 1% to 3% require hospitalisation for these complications, he said. To help prevent infections, physicians typically prescribe a prophylactic course of antibiotics before the procedure.
Dr. Hu noted that the investigators used a personalised approach to prophylactic antibiotics in the patients undergoing the transrectal biopsy procedure. Rather than giving the men a broad-spectrum antibiotic or multiple antibiotics, they matched the antibiotics to cultures obtained from the patient’s rectum during prostate exams before the procedure. This targeted antibiotic approach reduced the infection rate in those undergoing the traditional transrectal procedure substantially compared with the national infection rate for the procedure. Yet, they achieved a statistically significant reduction in infections in the transperineal group by eliminating infections altogether.
“Transperineal prostate biopsy makes a common diagnostic procedure safer for men,” said Dr Hu, who is also a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine. “It also eliminates the use of antibiotics, helping to reduce the emergence of antibiotic-resistant infections, a growing public health concern.”
Despite the promise of the new procedure, Dr. Hu acknowledged a few hurdles to making it more widely available to men in the United States. He explained that few physicians in the country have been trained in the perineal procedure. Additionally, he noted that US insurers pay the same amount for either procedure but the transperineal biopsy costs more and takes longer to perform, creating a financial disincentive for physicians to make the switch.
However, there is reason to think the status quo will change, Dr Hu said, noting the switch to transperineal prostate biopsies in Norway after a man died after a routine transrectal prostate biopsy. The change virtually eliminated biopsy-related infections and deaths in that country with the nationwide switch to transperineal biopsy, he said.
“There is a strong case to make the switch,” he said. “It will take time. But as more patients request the new procedure, we think it will become more widely available.”
Source: Weill Cornell Medicine