Researchers have developed a way to potentially reduce the toxic side-effects of CAR T cell immunotherapy, in findings that could overcome the pioneering treatment’s biggest limitation.
Their new study, reported in eLife, has come up with a way to identify a ‘goldilocks’ window that fine-tunes the cells used in the immunotherapy so that their activity is strong enough to eliminate the cancer but not so strong that they generate toxic side-effects.
Therapy provokes ‘perfect storm’
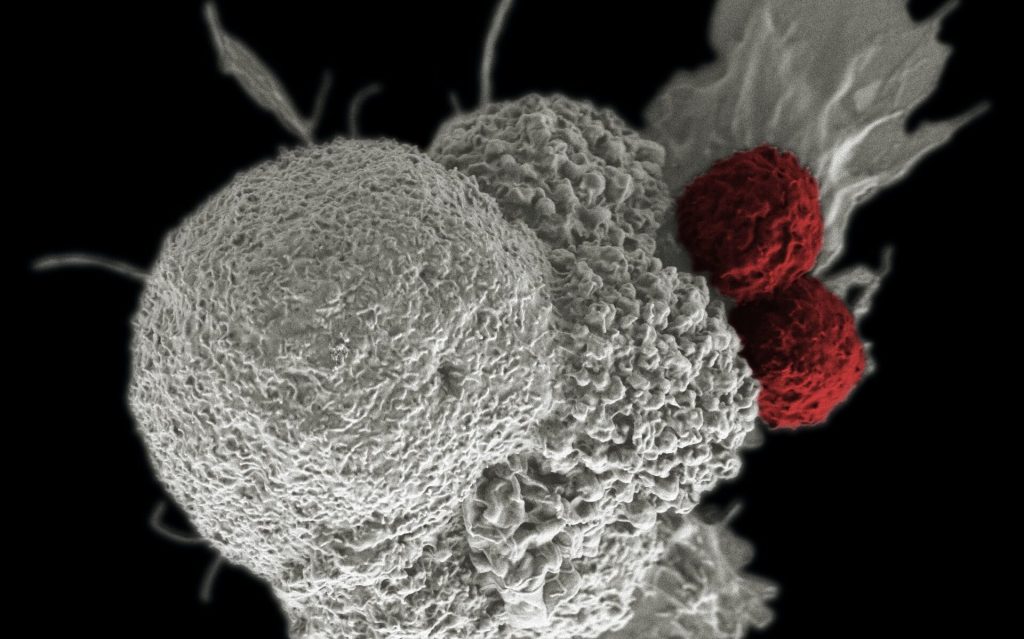
CAR T cell therapies involve collecting T cells from a cancer patient and supercharging the cells by individually re-engineering them in the laboratory. These enhanced cells are then put back into patients.
CAR T cell immunotherapy can be up to 90% effective in certain blood cancers, even curing some patients. But the treatment has harmful side-effects, with about 50% of patients experiencing dangerous complications.
The T cells are engineered to produce proteins on their surface called chimeric antigen receptors (CARs), which enable T cells to recognise and bind to specific proteins on the surface of cancer cells more efficiently.
Associate Professor Matthew Call said this synthetic sensor is what gives T cells the enhanced ability to attack and eliminate threats, like cancer cells.
“While putting these supercharged T cells into a patient with a high tumour burden can swiftly eradicate cancer cells, it also creates the perfect storm for an ongoing toxic response that can be harmful,” Associate Prof Call said.
There is currently no way of reliably predicting how strong CAR T cell therapy will be for a patient.
While previous studies have attempted to fine-tune T cells by targeting the end sections of the sensor, which either bind to the cancer cell or instruct the T cells to kill, the new research is the first to look at completely redesigning the middle part.
Researchers leveraged the computational expertise of the Weizmann Institute of Science to stitch together pieces of natural immune sensors with custom-designed synthetic elements, to generate new circuits that could be used to tune and assess variations of potency.
“Focusing on the connector fragment in the middle allows us to generate different versions of CARs that we know are stronger or weaker, enabling us to customise them to a patient’s potency requirements,” Associate Prof Call said.
“Being able to predictably tune this T cell activity significantly broadens our research, contrary to previous studies, because we are targeting something that exists in every immunotherapy scenario.
“For the first time, we can establish rules that will be applicable to any cancer where CAR T cell immunotherapy is being used.”
Enhanced treatment
Associate Prof Call said the ability to fine-tune T cells would dramatically reduce the number of patients experiencing severe side-effects from the treatment, which can include fever, high blood pressure and respiratory distress.
“CAR T cell therapy has proven effective in eradicating very advanced leukaemias and lymphomas, while also keeping the cancer at bay for many years – even after a patient has stopped taking cancer medication,” Associate Professor Call said.
“The therapy has incredible potential for cancer patients, but is currently used as a last resort due to these potentially severe side-effects.
“Our tools could lead to a fundamental rethink of the way CAR T cell therapy is offered by reducing a patient’s exposure risk to harmful side-effects. This would allow patients with a broad range of cancers to be given CAR T cell therapy far earlier in the treatment process.”
There are currently over 600 clinical trials of CAR T cell immunotherapy, with the treatment already being used for several blood cancers.
Researchers hope their new tool could be used to triage immunotherapy patients according to the potencies required in the early treatment phases, bringing the field closer to hitting that ‘goldilocks’ treatment window for many different cancers.
The next research phase will focus on progressing these findings into a clinical setting to see CAR T cell therapy used as a safer, first-line treatment.

