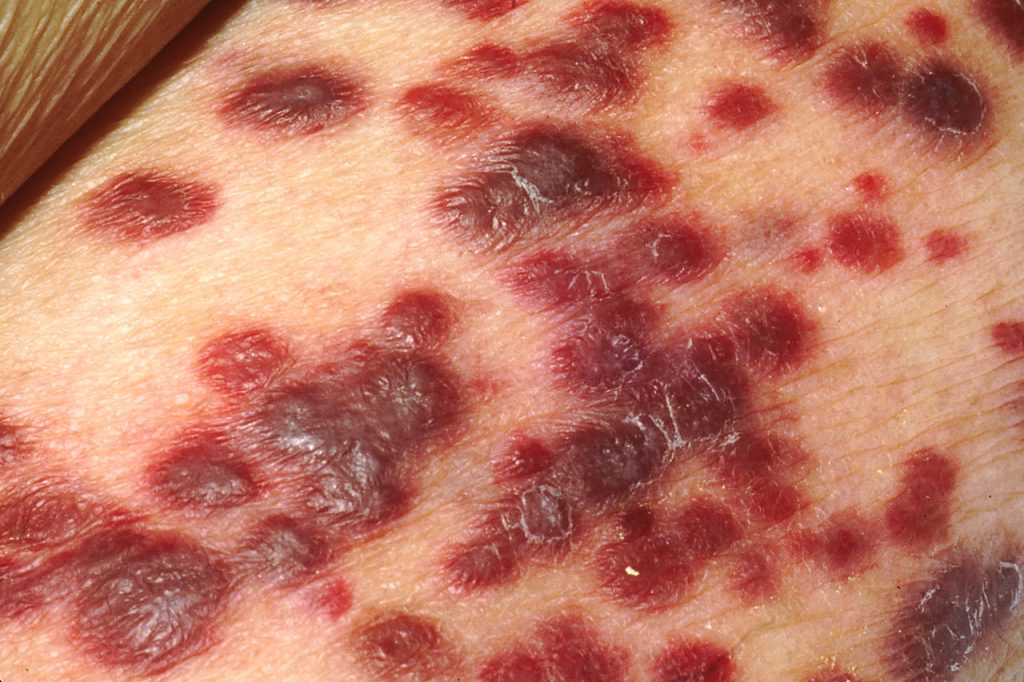
A study published in Cell Reports has identified a protein in the cancer cell’s nucleus as a critical agent keeping Kaposi sarcoma-associated herpesvirus (KSHV) dormant and hidden from the immune system. The virus, in the same family as Epstein-Barr virus, is linked to AIDS-related Castleman’s disease and cancers such as Kaposi sarcoma.
Up to 50% of the population in some parts of Africa are affected with KSHV, though not everyone with KSHV will develop Kaposi sarcoma. Those who do typically have a weakened immune system due to HIV infection, organ transplant, being older or other factors.
The introduction of antiretroviral therapy significantly reduced AIDS-related Kaposi sarcoma prevalence in Western countries; however, in sub-Saharan Africa, the disease continues to have a poor prognosis.
On entry into a human cell the virus causes a hidden infection in the nucleus: the virus simply latches onto parts of the cell’s chromosomes without replicating.
Researchers studied KSHV’s latent-lytic switch, a process in which the virus exits its dormancy state to replicate in the host cell. This replication phase, called the lytic cycle, ends with the disintegration of the cell and the release of the viruses, infecting neighbouring cells.
“The virus likes to stay silent as long as possible to avoid being detected by the body’s immune system,” said Professor Yoshihiro Izumiya, the study’s senior author.
The team sought to understand the mechanisms behind this latent-lytic switch and the role the host cell environment played in this process.
“Where the virus latches onto the host cell, how it manages to stay dormant, and what triggers its activation were very exciting and important puzzles to solve,” Prof Izumiya said.
The study identified where the virus genome could be found on the host genome.
Izumiya and his team profiled and analysed chromosomal interactions on three cancer cell lines naturally infected with KSHV, locating the virus’s preferred chromosome docking sites. The binding patterns, similar among the three cancer cell lines, showed a nuclear ecosystem that can attract and help keep the virus in its silent form.
The team also found that CHD4 (chromodomain helicase DNA binding protein 4) binds to the virus’s genomic elements. CHD4, a protein in the host cell’s chromosomes, suppresses the work of the gene responsible for viral replication. The study showed that CHD4 is a key regulator of the KSHV latency-lytic switch.
“The location where the virus genome attaches to the host chromosome is not random,” said Ashish Kumar, a postdoctoral researcher in Izumiya Lab and the paper’s first author. “Without having enriched CHD4 protein, the virus starts to replicate, kicking in a cell destructive mode. For the virus to select CHD4 among many other host proteins, CHD4 must play a unique and important role in host cells.”
Virology can help identify cellular proteins essential for cell homeostasis. Over millions of years, the virus’s genome developed to encode or assemble a small number of very efficient proteins, which strategically connect to host cell proteins to keep viral chromatin dormant and impact the host cell’s tumour suppression function.
“We used virology as an entry point to shed light on the function of CHD4 in gene regulation in general. During virus-host co-evolution, KSHV cleverly learned to hijack host proteins that can help keep the gene responsible for viral replication dormant.”
The researchers found a viral protein which could serve as the basis for a replication inhibitor. Since CHD4 is critical for cancer cell growth in a variety of cancers, they hope this virus-host interaction could inform cancer treatment research.

