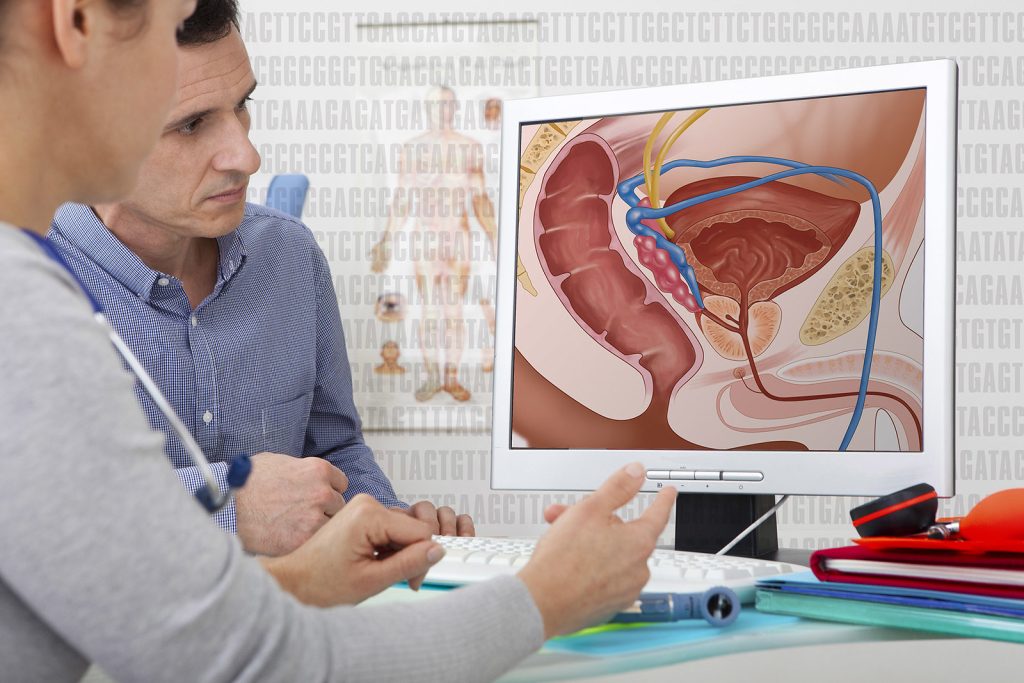
Researchers have developed a new urine-based test that addresses a major problem in prostate cancer: how to separate the slow-growing form of the disease unlikely to cause harm from more aggressive cancer that needs immediate treatment.
The test, called MyProstateScore2.0, or MPS2, looks at 18 different genes linked to high-grade prostate cancer. In multiple tests using urine and tissue samples from men with prostate cancer, it successfully identified cancers classified as Gleason 3+4=7 or Grade Group 2 (GG2), or higher. These cancers are more likely to grow and spread compared to Gleason 6 or Grade Group 1 prostate cancers, which are unlikely to spread or cause other impact. More than one-third of prostate cancer diagnoses are this low-grade form. Gleason and Grade Group are both used to classify how aggressive prostate cancer is.
Results from the University of Michigan Rogel Cancer Center-led study are published in JAMA Oncology.
“Our standard test is lacking in terms of its ability to clearly pick out those who have significant cancer. Twenty years ago, we were looking for any kind of cancer. Now we realise that slow-growing cancer doesn’t need to be treated. All of a sudden, the game changed. We went from having to find any cancer to finding only significant cancer,” said co-senior study author John T. Wei, M.D., David A. Bloom Professor of Urology at Michigan Medicine.
Prostate-specific antigen, or PSA, remains the linchpin of prostate cancer detection. MPS2 improves upon a urine-based test developed by the same U-M team nearly a decade ago, following a landmark discovery of two genes that fuse to cause prostate cancer. The original MPS test, which is used today, looked at PSA, the gene fusion TMPRSS2::ERG, and another marker called PCA3.
“There was still an unmet need with the MyProstateScore test and other commercial tests currently available. They were detecting prostate cancer, but in general they were not doing as good a job in detecting high-grade or clinically significant prostate cancer. The impetus for this new test is to address this unmet need,” said co-senior author Arul M. Chinnaiyan, M.D., Ph.D., director of the Michigan Center for Translational Pathology. Chinnaiyan’s lab discovered the T2::ERG gene fusion and developed the initial MPS test.
“If you’re negative on this test, it’s almost certain that you don’t have aggressive prostate cancer,” said Chinnaiyan, S. P. Hicks Endowed Professor of Pathology and professor of urology at Michigan Medicine.
Moreover, MPS2 was more effective at helping patients avoid unnecessary biopsies. While 11% of unnecessary biopsies were avoided with PSA testing alone, MPS2 testing would avoid up to 41% of unnecessary biopsies.
“Four of 10 men who would have a negative biopsy will have a low risk MPS2 result and can confidently skip a biopsy. If a man has had a biopsy before, the test works even better,” Wei explained.
For example, a patient may get a prostate biopsy due to an elevated PSA, but no cancer is detected. The patient is followed over time and if his PSA inches up, he would typically need another biopsy.
“In those men who have had a biopsy before and are being considered for another biopsy, MPS2 will identify half of those whose repeat biopsy would be negative. Those are practical applications for patients out there. Nobody wants to say sign me up for another biopsy. We are always looking for alternatives and this is it,” Wei said.

