A new discovery, which was published in Nature Cell Biology, reveals how genetic material can escape mitochondria, prompting the body to launch a damaging immune response, setting off diseases such as lupus and rheumatoid arthritis. By developing therapies to target this process, doctors may one day be able to stop the harmful inflammation and prevent the toll it takes on our bodies.
“When mitochondria don’t correctly replicate their genetic material, they try to eliminate it. However, if this is happening too often and the cell can’t dispose of all of it, it can cause inflammation, and too much inflammation can lead to disease, including autoimmune and chronic diseases,” said researcher Laura E. Newman, PhD, of the University of Virginia School of Medicine. “Now that we are beginning to understand how this inflammation starts, we might be able to prevent this process, with the ultimate goal of limiting inflammation and treating disease.”
Powering inflammation
Mitochondria have their own set of genetic material, separate from the DNA that serves as the operating instructions for our cells. Scientists have known that this mitochondrial DNA, known as mtDNA, can escape into our cells and cause inflammation. But exactly what caused this has been a mystery until now.
“We knew that mtDNA was escaping mitochondria, but how was still unclear,” said Gerald Shadel, PhD, director of the San Diego-Nathan Shock Center of Excellence in the Basic Biology of Aging at the Salk Institute. “Using imaging and cell biology approaches, we’re able to trace the steps of the pathway for moving mtDNA out of the mitochondria, which we can now try to target with therapeutic interventions to hopefully prevent the resulting inflammation.”
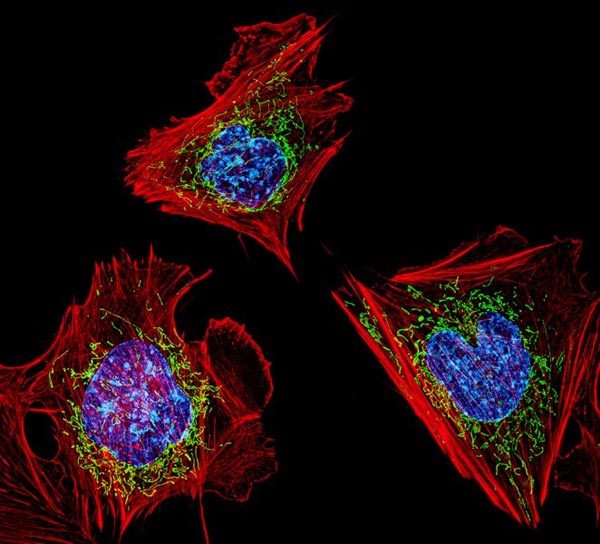
Shadel and Newman, then a postdoctoral researcher in Shadel’s lab, and their collaborators used sophisticated imaging techniques to determine what was happening inside the leaky mitochondria. They found that the leak was triggered by a malfunction in mtDNA replication. This caused the accumulation of protein masses caused nucleoids.
To try to fix this problem, the cell containing the faulty mitochondrion begins to export the excess nucleoids to its cellular trash bins. But the trash bins, called endosomes, can become overwhelmed by the volume of debris, the scientists found. These overburdened endosomes respond by releasing mtDNA into the cell — in short, the trash can overflows.
“We had a huge breakthrough when we saw that mtDNA was inside of a mysterious membrane structure once it left mitochondria. After assembling all of the puzzle pieces, we realised that structure was an endosome,” Newman said. “That discovery eventually led us to the realisation that the mtDNA was being disposed of and, in the process, some of it was leaking out.”
The cell responds to this hazardous waste spill by flagging the nucleoids as foreign DNA, like a virus, and launches an immune response that results in harmful inflammation, the scientists determined.
“Using our cutting-edge imaging tools for probing mitochondria dynamics and mtDNA release, we have discovered an entirely novel release mechanism for mtDNA,” said researcher Uri Manor, PhD, former director of the Waitt Advanced Biophotonics Core at Salk and current assistant professor at UC San Diego. “There are so many follow-up questions we cannot wait to ask, like how other interactions between organelles control innate immune pathways, how different cell types release mtDNA, and how we can target this new pathway to reduce inflammation during disease and aging.”
Newman will continue to seek these answers in her new role at the UVA School of Medicine’s Department of Cell Biology. “We want to understand the physiological and disease contexts where this process can become activated,” she said. “For example, many viruses attack mitochondria during infection, so we will be testing whether mitochondria purposely use this pathway to sound the alarm against invading viruses, and whether over-reliance on this pathway to fight off infection can later trigger chronic diseases.”

