
In a clinical trial, nearly every one of the 112 patients with mismatch repair-deficient (dMMR) colon cancer achieved a pathologic response with just two cycles of neoadjuvant immunotherapy, prompting a presentation panellist to describe it as “striking data” – though a note of caution was given.
Patients in the NICHE-2 single-arm study received PD-1 plus CTLA-4 blockade – nivolumab plus ipilimumab, and successfully underwent surgical resection, 98% on time, meeting the study’s primary safety endpoint, reported Myriam Chalabi, MD, at the at the European Society for Medical Oncology (ESMO) annual congress.
And with a median follow-up of 13.1 months, the disease-free survival (DFS) rate was 100%, said Dr Chalabi of the Netherlands Cancer Institute in Amsterdam. She pointed out that, by this point, the expected rate for this patient population was about 15%. The primary efficacy endpoint for the trial is DFS at 3 years, with success defined as a rate of 93% (data are expected next year).
A standing ovation erupted when Dr Chalabi displayed the waterfall plot showing the depth of pathologic response with just four weeks of nivolumab plus ipilimumab.
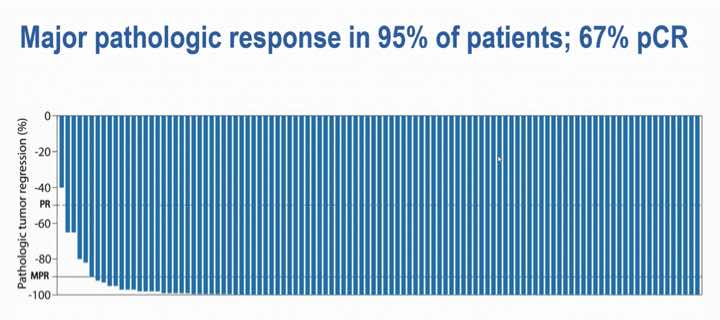
Among the 107 patients evaluable for efficacy, all but one had a pathologic response, 95% had a major pathologic response (MPR), and 67% had a pathologic complete response (pCR), ie no residual viable tumour in both the primary tumour bed and lymph nodes.
“As you can appreciate, the pathologic regression observed was near-complete or complete in almost all patients,” she said.
In contrast, pathologic response rates in the range of 5% to 7% for this population have been shown in prior trials involving neoadjuvant chemotherapy, said Chalabi.
Some 10–15% of colon cancers are classified as dMMR, she explained, which are highly sensitive to immune checkpoint inhibitors, where a number of agents have been approved for the metastatic setting.
The first to comment in Q&A, Alexander Eggermont, MD, PhD, stressed the potential impact of the findings, saying that patients with dMMR tumours scheduled for resection “should be taken off the surgical program.”
“They should be sent to the medical oncologist for the first dose of ipi/nivo,” he said. “We will live the day that they will not undergo surgery anymore after these schedules – that’s the next step.”
A multicentre, single-arm study, NICHE-2 enrolled 112 patients with previously untreated non-metastatic dMMR colon cancer undergoing surgery. The first cycle of neoadjuvant treatment included ipilimumab (1mg/kg) and nivolumab (3mg/kg). The second cycle, given two weeks later, was limited to nivolumab alone. After the first dose, median time to surgery was 5.4 weeks.
The trial defined pathologic response as 50% or less residual viable tumour; MPR was defined as 10% or less residual viable tumour, and included patients with pCRs in the primary tumour but viable tumour in the lymph nodes.
The median age of patients was 60, and 58% were women. About three-fourths had high-risk stage III disease, 13% had low-risk stage III disease, and 13% had stage I/II disease. About half had radiologic high-risk disease (both T4 and N2), said Dr Chalabi, and abdominal wall involvement was common.
When asked whether randomised data would be needed to make this approach standard, Dr Chalabi pointed out the group with T4 tumours.
“I wouldn’t want to randomise those patients,” she said. “Surgeons usually would prefer to have some type of downstaging before continuing on to surgery in order to increase the chances of achieving tumour-free resection margins and also to limit the extent of surgery needed to achieve that.”
The case for randomisation is stronger in earlier disease, she said, but if recurrences can be prevented even in stages where recurrence is more rare, such as stage II tumours (about 10% at 3 years), “we’re curing 10% more patients.”
A little less than a third of patients had Lynch syndrome, and pCRs were more frequent in this subset (78% vs 58% in those with sporadic tumours). Immune-related adverse events (AEs) were reported in 61% of patients, with 4% being grade 3/4.
When the prospect of a NICHE-3 trial came up, Dr Chalabi said that it ideally would have been an international study to validate the approach. However, a subsequent trial is being developed and will likely involve nivolumab plus anti-LAG-3 relatlimab, “which is a shame,” she noted.
“If we do get similar responses with nivolumab and anti-LAG-3, then that may be an avenue to test organ-sparing approaches with that combination in this population,” she added.
Source: MedPage Today

