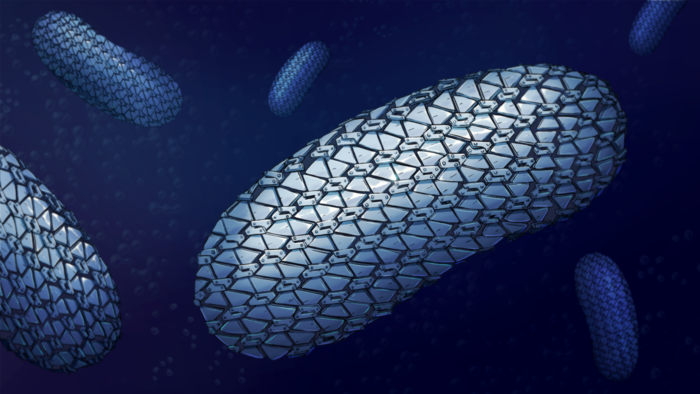
The spectacular structure of the protective armour of C. difficile has been revealed for the first time showing the close-knit yet flexible outer layer – like a mediaeval knight’s chain mail.
This tight arrangement keeps molecules from getting in and provides a new target for future treatments, according to the scientists who have uncovered it.
Published in Nature Communications, the team of scientists outlined the structure of the main protein, SlpA, that forms the links of the chain mail and how they link up to form a pattern and create this flexible armour.
One of the many ways that Clostridioides difficile has to protect itself from antibiotics is a special layer that covers the cell of the whole bacteria – the surface layer or S-layer. This flexible armour protects against the entry of drugs or molecules released by our immune system to fight bacteria.
Using a combination of X-ray and electron crystallography, the team determined the structure of the proteins and their arrangement.
Corresponding author and lead researcher Dr Paula Salgado said: “I started working on this structure more than 10 years ago, it’s been a long, hard journey but we got some really exciting results! Surprisingly, we found that the protein forming the outer layer, SlpA, packs very tightly, with very narrow openings that allow very few molecules to enter the cells. S-layer from other bacteria studied so far tend to have wider gaps, allowing bigger molecules to penetrate. This may explain the success of C.diff at defending itself against the antibiotics and immune system molecules sent to attack it.
“Excitingly, it also opens the possibility of developing drugs that target the interactions that make up the chain mail. If we break these, we can create holes that allow drugs and immune system molecules to enter the cell and kill it.”
Antimicrobial resistance (AMR), a growing problem, was declared by WHO as one of the top 10 global public health threats facing humanity.
One of the many bacteria that have evolved resistance to antibiotics, C. diff infects the human gut and is resistant to all but three current drugs. Antibiotics only compound the problem, as the good bacteria in the gut are killed alongside those causing an infection and, as C. diff is resistant, it can grow and cause diseases ranging from diarrhoea to death due to massive lesions in the gut. Since the only way to treat C.diff is to take antibiotics, it creates a vicious cycle of recurrent infections.
This knowledge could lead to the development of C. diff specific drugs that break the protective layer, creating holes to allow drug molecules to penetrate and kill the cell.
Dr Rob Fagan, who helped carry out the electron crystallography work, said: “We’re now looking at how our findings could be used to find new ways to treat C. diff infections such as using bacteriophages to attach to and kill C. diff cells – a promising potential alternative to traditional antibiotic drugs.”
Source: EurekAlert!

