A group of newly discovered bacteriophages named after the UK village of Colney could help combat C. difficile infections.
Clostridioides difficile, or C. diff, is a species of bacteria that infects the human gut. It can become a major problem when our normal gut microbes are impaired, most commonly during a course of antibiotics. This leads to an overgrowth of C. diff, with toxins it produces causing diarrhoea and severe inflammation.
Treatment involves further courses of antibiotics, but relapse and recurrent infections are common. The strains are becoming more resistant to antibiotics and causing more severe illness.
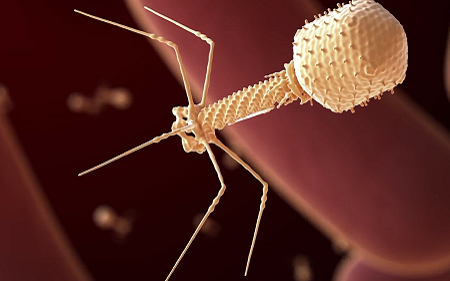
This prompted researchers in Norwich to look for the bacteria’s natural enemy, bacteriophages. They screened 27 different C. diff strains for any bacteriophages, finding one, which they called ΦCD27 (phiCD27). Genome sequencing confirmed this phage had not been discovered before. In fact, the members of the International Committee on Taxonomy of Viruses (ICTV) decided it was genetically distinct enough to form a new group, or genus of phages.
The ICTV decided to name the new genus Colneyvirus, the Colney parish address of the Institute of Food Research (IFR, now part of Quadram Institute), where it was first discovered.
Like normal viruses, phages reproduce by injecting their genetic material into bacteria, making viral copies using the host’s own machinery. Using enzymes called endolysins, they destroy the bacterial cell wall and escape.
The researchers extracted the gene for ΦCD27’s endolysin and put it into another bacterium, E. coli so that they could produce and purify the endolysin. It was proven active against 30 different C. diff strains, including hypervirulent strains behind the current epidemic. It also didn’t affect other common bacterial species in the human gut microbiome.
”This phage and the endolysin encoded by its genome can provide a targeted approach to combat C. diff infections, in contrast to use of broad spectrum antibiotics that cause collateral damage by inhibiting other members of the gut bacterial population” said Professor Arjan Narbad, Group Leader at the Quadram Institute.
However, to be effective the endolysins need to be delivered into the gut, so the team also put the gene into a strain of lactic acid bacteria that has previously been used to deliver proteins and vaccines to the gut.
The research team believes this could serve as the basis for future new treatments C. diff. The system needs more work, but in the battle against this bacterial pandemic, the colneyvirus could be a vital ally.
Source: Quadram Institute

